FISH for Microbial Detection: A Comprehensive Guide from Principles to Clinical Applications
This article provides a comprehensive overview of Fluorescence in Situ Hybridization (FISH) for the detection, identification, and localization of microorganisms.
FISH for Microbial Detection: A Comprehensive Guide from Principles to Clinical Applications
Abstract
This article provides a comprehensive overview of Fluorescence in Situ Hybridization (FISH) for the detection, identification, and localization of microorganisms. Tailored for researchers, scientists, and drug development professionals, it covers the foundational principles and evolution of FISH technology, detailed methodological protocols for diverse applications, practical troubleshooting strategies for common challenges, and rigorous validation frameworks alongside comparative analysis with other diagnostic techniques. By synthesizing current research and practical insights, this guide serves as an essential resource for implementing and optimizing FISH in both research and clinical microbiology settings, highlighting its unique advantages in culture-free, in-situ analysis of complex microbial communities.
The Foundation of FISH: Principles, Probes, and Technological Evolution in Microbiology
Fluorescence in situ hybridization (FISH) represents a cornerstone molecular cytogenetic technique that enables the direct visualization and localization of specific nucleic acid sequences within morphologically preserved chromosomes, cells, or tissue sections [1]. The fundamental principle underpinning FISH involves the hybridization of fluorescently labeled DNA or RNA probes to complementary target sequences, allowing researchers to detect the presence, absence, and spatial organization of genetic elements within a biological context [2]. Since its development in the early 1980s, FISH has evolved into an indispensable tool for genetic counseling, medicine, species identification, and increasingly, microbial detection research [1].
For investigators studying microbial communities, FISH offers a powerful approach to identify, quantify, and spatially localize microorganisms within complex samples without the need for cultivation. This application note details the core principles of probe hybridization, provides detailed protocols adaptable for microbial detection, and presents key reagent solutions to facilitate effective research planning and implementation.
Core Principles of FISH
The Hybridization Process
The essence of FISH technology centers on the precise molecular recognition between a labeled nucleic acid probe and its complementary target sequence within a cellular environment. This process relies on the fundamental principle of base pairing (A-T and G-C for DNA; A-U and G-C for RNA) under specific hybridization conditions [1] [2]. The technique preserves the structural integrity of the sample while providing critical information about genetic architecture that would be lost with nucleic acid extraction methods.
Probes are typically designed as single-stranded DNA or RNA fragments complementary to a nucleotide sequence of interest [1]. For microbial detection, these are often targeted to species-specific 16S rRNA sequences due to their phylogenetic significance and high copy number within bacterial cells. When the probe encounters its complementary target under appropriate conditions, it forms a stable double-stranded hybrid through hydrogen bonding, which can then be visualized via fluorescence microscopy due to the attached fluorophore.
Probe Design and Labeling Strategies
FISH probes can be categorized based on their target scope and composition:
- Locus-specific probes hybridize to particular genomic regions and are essential for detecting specific microbial taxa or functional genes [1].
- Whole-chromosome painting probes consist of mixtures that bind along entire chromosomes, less commonly used in microbial research [1].
- Oligonucleotide probes (typically 20-50 nucleotides) offer high specificity and are widely employed for microbial rRNA targeting [1].
Probe labeling can be achieved through various methods, including nick translation, PCR with tagged nucleotides, or in vitro transcription for RNA probes [1] [3]. Modern FISH Tag kits utilize enzymatic incorporation of amine-modified nucleotides followed by chemical labeling with amine-reactive dyes, resulting in higher incorporation efficiency and improved signal-to-noise ratios [3].
Table 1: Common Fluorophores Used in FISH Applications
| Fluorophore | Excitation Max (nm) | Emission Max (nm) | Common Applications |
|---|---|---|---|
| DAPI | 358 | 461 | Nuclear counterstain |
| FITC | 495 | 519 | Standard labeling (green) |
| Alexa Fluor 555 | 555 | 565 | Standard labeling (red) |
| Texas Red | 589 | 615 | Standard labeling (far red) |
| Alexa Fluor 647 | 650 | 668 | Standard labeling (near IR) |
| Cy3 | 554 | 568 | Standard labeling (orange) |
Detailed FISH Protocol for Microbial Detection
This protocol adapts established FISH methodologies for the detection and visualization of microorganisms in sample specimens, incorporating critical steps for optimal hybridization and signal detection [1] [2] [3].
Sample Preparation and Fixation
- Sample Collection: Collect microbial cells from appropriate sources (culture, environmental samples, biofilms). For planktonic cells, concentrate via centrifugation at 5,000 × g for 10 minutes.
- Fixation: Resuspend cell pellet in fresh fixative solution (4% formaldehyde or paraformaldehyde in PBS) and incubate for 1-4 hours at 4°C. This step preserves cellular morphology and prevents degradation.
- Permeabilization: Pellet cells (5,000 × g, 5 min), wash with PBS, and treat with permeabilization solution (0.1% Triton X-100 or Tween-20 in PBS) for 10-15 minutes. This creates pores for probe penetration.
- Slide Preparation: Apply fixed cell suspension onto clean microscope slides and air dry. Dehydrate through an ethanol series (50%, 80%, 96%; 3 min each) and air dry completely.
Hybridization Procedure
- Probe Preparation: Dilute the fluorescently labeled probe in appropriate hybridization buffer to working concentration (typically 2-10 ng/μL). Specific probe sequences should target microbial 16S rRNA or other signature genes.
- Denaturation: Apply probe solution to sample area, add coverslip, and seal edges with rubber cement. Denature slide and target DNA simultaneously on a preheated hotplate or hybridizer at 75°C for 2-5 minutes [2].
- Hybridization: Immediately transfer slides to a humidified chamber and incubate at appropriate hybridization temperature (typically 37-46°C, depending on probe design) for 2-16 hours (often overnight) to allow specific probe binding [2].
Post-Hybridization Washing and Detection
- Stringency Washes: Remove coverslips carefully and wash slides in pre-warmed wash buffer (0.4× SSC at 72°C for 2 minutes) to remove nonspecifically bound probes [2].
- Secondary Wash: Perform additional wash in room temperature solution (2× SSC with 0.05% Tween-20 for 30 seconds) [2].
- Counterstaining and Mounting: Apply appropriate counterstain (e.g., DAPI for DNA) in antifade mounting medium and add coverslip. For signal amplification, proceed with tyramide signal amplification (TSA) using SuperBoost kits before counterstaining [3].
Microscopy and Analysis
Visualize samples using epifluorescence or confocal microscopy equipped with appropriate filter sets for the fluorophores used. For microbial quantification, count specific signals in multiple random fields until at least 100-200 cells are enumerated. Analyze spatial distribution patterns when examining complex samples like biofilms.
Advanced FISH Methodologies
Single-Molecule RNA FISH (smFISH)
For detecting and quantifying individual mRNA molecules in microbial cells, smFISH (also known as Stellaris RNA FISH) employs multiple short singly labeled oligonucleotide probes (typically up to 48) binding to a single transcript [1]. This approach provides sufficient localized fluorescence to detect and localize each target mRNA molecule with high precision, enabling studies of gene expression heterogeneity in microbial populations at single-cell resolution.
Tyramide Signal Amplification (TSA)
For detecting low-abundance targets or enhancing weak signals in microbial detection, TSA systems (such as SuperBoost kits) offer sensitivity 10-200 times greater than standard FISH methods [3]. This enzyme-mediated methodology deposits multiple fluorophore-labeled tyramide molecules at the probe binding site, dramatically amplifying signal intensity for challenging targets like low-copy-number genes or small microorganisms.
Table 2: Signal Amplification Kits for Low-Abundance Targets
| Kit Type | Sensitivity Gain | Key Components | Application Scope |
|---|---|---|---|
| Standard TSA | 10-50x | HRP-streptavidin, Tyramide-dye | Routine signal enhancement |
| SuperBoost Kits | 10-200x | Poly-HRP, Alexa Fluor tyramides | Very rare or low-abundance targets |
| FISH Tag Kits | N/A | Amine-modified nucleotides, Succinimidyl ester dyes | High signal-to-noise multiplexing |
Research Reagent Solutions
Successful FISH experimentation requires specific reagents and systems optimized for particular sample types and detection requirements. The following table outlines essential solutions for implementing FISH in microbial detection research.
Table 3: Essential Research Reagents for FISH Experiments
| Reagent Category | Specific Examples | Function & Application Notes |
|---|---|---|
| Fixation Solutions | 4% Formaldehyde, Paraformaldehyde (PFA) in PBS | Preserves cellular morphology and prevents nucleic acid degradation |
| Permeabilization Agents | Triton X-100 (0.1%), Tween-20, Proteinase K | Creates membrane pores for probe entry; concentration optimization critical |
| Hybridization Buffers | SSC-based buffers with formamide, dextran sulfate | Maintains pH and ionic strength during hybridization; formamide reduces melting temperature |
| Labeling Systems | FISH Tag DNA/RNA Kits, Nick translation kits | Enzymatic incorporation of fluorophores or haptens into nucleic acid probes |
| Signal Amplification | SuperBoost Tyramide Kits, TSA Plus Systems | Dramatically enhances detection sensitivity for low-abundance targets |
| Detection Fluorophores | Alexa Fluor dyes (488, 555, 594, 647) | Photostable fluorophores with high quantum yields for multiplex detection |
| Counterstains | DAPI, Hoechst 33258, Propidium Iodide | Provides structural context by staining nucleic acids |
| Mounting Media | Antifade reagents (Vectashield, ProLong) | Preserves fluorescence during microscopy and storage |
Troubleshooting and Optimization
Successful FISH implementation requires careful attention to potential technical challenges:
- High Background Fluorescence: Increase stringency of post-hybridization washes by raising temperature or decreasing salt concentration; reduce probe concentration; ensure complete removal of unbound probe [1] [2].
- Weak or Absent Signal: Extend hybridization time; increase probe concentration; incorporate signal amplification; verify probe design and labeling efficiency; check fluorophore integrity [3].
- Autofluorescence in Samples: Employ ethanol washes to reduce autofluorescence; utilize fluorophores with emission spectra distinct from sample autofluorescence; consider chemical treatments to reduce autofluorescence [1].
- Poor Cellular Morphology: Optimize fixation conditions (type, concentration, duration); avoid over-digestion during permeabilization steps [4].
For quantitative analysis, ensure consistent hybridization conditions across experiments and include appropriate positive and negative controls. Newer computational approaches for image analysis can further enhance the objectivity and reproducibility of FISH-based microbial detection and quantification.
Fluorescence In Situ Hybridization (FISH) represents one of the most significant technological evolutions in molecular cytogenetics, enabling researchers to visualize and map genetic material within intact cells and tissues. This revolutionary technique has transformed our understanding of genomic organization, gene expression, and chromosomal abnormalities across diverse fields including microbiology, oncology, and genetic disease research. The journey from radioactive detection methods to modern fluorescent approaches marks a critical advancement in diagnostic precision, safety, and multiplexing capability. Within microbial detection research specifically, FISH has emerged as an indispensable tool for identifying and characterizing microorganisms in their native environments without the need for cultivation [5]. The development of FISH probes has progressed from simple single-color DNA detection to sophisticated multiplex assays capable of simultaneously visualizing multiple genetic targets, with the global FISH probe market reflecting this technological expansion through substantial growth from USD 1.14 billion in 2025 to a projected USD 2.27 billion by 2034 [6]. This application note details the historical development, current methodologies, and practical implementation of FISH technologies, with particular emphasis on applications within microbial detection research.
Technological Evolution: From Radioactive to Fluorescent Detection
The development of in situ hybridization technologies has traversed a remarkable path from radioactive isotopes to sophisticated fluorescent detection systems. The earliest ISH methodologies utilized radioactive labels such as tritium (³H) or phosphorus (³²P), which provided the sensitivity required for initial detection of specific DNA and RNA sequences but posed significant limitations including safety hazards, long exposure times (often requiring weeks to months), and poor spatial resolution. The transition to non-radioactive detection methods began in the 1980s with the introduction of hapten-labeled probes detected through enzymatic reactions, but the true revolution arrived with the implementation of fluorescent labeling.
The advent of FISH technology addressed multiple limitations simultaneously by incorporating fluorophore-conjugated nucleotides directly into nucleic acid probes. This innovation provided researchers with a safer, faster, and more versatile detection platform that preserved cellular morphology while allowing direct visualization through fluorescence microscopy. The initial single-color FISH protocols quickly evolved into dual-color systems, enabling the simultaneous detection of two genetic targets. Contemporary FISH technologies now support multiplex assays with capacity for dozens of simultaneous detections through sophisticated probe design and spectral imaging [7].
The technological progression of FISH has been characterized by several key developments:
- Probe Design Evolution: Advancements shifted from large genomic clones (cosmids, BACs) to shorter oligonucleotides (OligoPaint), improving resolution and specificity [8]
- Signal Amplification Systems: Development of tyramide signal amplification (TSA) and hybridization chain reaction (HCR) dramatically increased detection sensitivity [8]
- Multiplexing Capabilities: Introduction of spectral karyotyping (SKY) and multiplex FISH (M-FISH) enabled complete chromosome visualization [7]
- Automation and AI Integration: Recent developments include automated imaging platforms and AI-assisted analysis tools like U-FISH, which provides universal spot detection across diverse FISH datasets [9]
Table 1: Evolution of Key FISH Probe Types and Their Applications
| Probe Type | Historical Period | Key Characteristics | Primary Applications |
|---|---|---|---|
| Radioactive ISH Probes | 1969-1980s | Used ³H or ³²P isotopes; long exposure times; safety concerns | Initial gene mapping; viral detection |
| First-Generation FISH | 1980s-1990s | Single fluorophore labels; limited multiplexing | Chromosome enumeration; gene localization |
| DNA Probes | 1990s-Present | Stable hybridization; 45% market share in 2024 [6] | Oncology diagnostics; genetic disease detection |
| RNA Probes | 2000s-Present | Detection of gene expression; fastest growing segment [6] | Microbial identification; transcriptomics |
| Multiplex FISH | 2010s-Present | Simultaneous detection of multiple targets | Complex chromosomal rearrangements |
| Live-FISH | 2020s-Present | Maintenance of cell viability during hybridization | Targeted cultivation of soil microbiomes [5] |
Current Market Landscape and Quantitative Analysis
The FISH probe market demonstrates robust growth driven by increasing applications in clinical diagnostics and research. Current market analysis reveals a compound annual growth rate (CAGR) of 7.93% from 2025 to 2034, with the market value projected to reach approximately USD 2.27 billion by 2034 [6]. This expansion reflects the growing integration of FISH technologies into routine clinical practice, particularly in oncology and genetic disorder diagnostics.
The market distribution by probe type shows DNA probes maintaining dominance with 45% market share in 2024, while RNA probes represent the fastest-growing segment due to increasing applications in gene expression analysis and spatial transcriptomics [6]. From a technological perspective, recent developments have included the incorporation of quantum dots as fluorescent labels, which offer superior photostability and narrow emission spectra ideal for multiplex applications [6]. The label type segmentation shows fluorescent dyes currently leading the market with 50% share, while quantum dots are anticipated to expand at a significant CAGR from 2025 to 2034 [6].
Application analysis reveals oncology as the dominant segment with 55% market share in 2024, largely driven by the critical role of FISH in detecting oncogenic mutations, chromosomal translocations, and gene amplifications essential for diagnosis, prognosis, and therapy selection [6]. The prenatal and genetic disorder diagnosis segment is expected to grow at the fastest rate during the forecast period, reflecting increasing implementation of FISH in non-invasive prenatal testing and neonatal screening [6].
Geographically, North America dominated the FISH probe market with approximately 47% share in 2024, while the Asia Pacific region is expected to register the fastest growth rate due to rising prevalence of target diseases, improving healthcare facilities, and growing demand for in vitro diagnostic testing [6] [10]. This regional distribution corresponds with healthcare infrastructure development and research funding patterns.
Table 2: Global FISH Probe Market Segmentation and Growth Projections
| Segmentation Category | 2024 Market Share | Projected CAGR (2025-2034) | Key Growth Drivers |
|---|---|---|---|
| By Probe Type | |||
| DNA Probes | 45% [6] | Stable growth | Cancer diagnostics; genetic disorder detection |
| RNA Probes | Smaller share | Fastest growing [6] | Gene expression analysis; spatial transcriptomics |
| By Application | |||
| Oncology | 55% [6] | 7.93% overall market CAGR [6] | Precision medicine; targeted therapies |
| Prenatal & Genetic Disorders | Smaller share | Fastest growing segment [6] | Non-invasive prenatal testing; newborn screening |
| By End User | |||
| Hospitals & Diagnostic Centers | 50% [6] | Steady growth | Routine clinical diagnostics |
| Research & Academic Institutes | Smaller share | Fastest growing [6] | Spatial-omics; basic research |
| By Region | |||
| North America | 47% [6] | Stable growth | Advanced healthcare infrastructure |
| Asia Pacific | Smaller share | Fastest growing [6] | Improving healthcare facilities |
Application in Microbial Detection Research
FISH technologies have revolutionized microbial detection research by enabling the identification, quantification, and spatial localization of microorganisms within complex environmental samples without requiring cultivation. The application of FISH in microbiology has been particularly valuable for studying uncultivable microorganisms, which represent the majority of microbial diversity in most environments. The technique has been successfully applied to diverse fields including environmental microbiology, human microbiome research, and industrial process monitoring.
Recent advances in Live-FISH have further expanded applications by maintaining cell viability throughout the hybridization process, enabling subsequent cultivation of targeted microorganisms. A 2025 study evaluating Live-FISH on soil microbiomes demonstrated that, while the procedure reduced the number of viable cells by approximately one order of magnitude, 501 amplicon sequence variants (ASVs) retained viability and could serve as targets for future cultivation efforts [5]. The study revealed taxon-specific effects, with Planctomycetota and Bacillota demonstrating greater resilience to Live-FISH treatment compared to Acidobacteriota, which were reduced by five orders of magnitude [5]. This selective impact highlights the importance of protocol optimization for specific target microorganisms.
The development of more permeable probe designs and gentler hybridization conditions has been crucial for microbial applications, as many environmental microorganisms possess robust cell walls that limit probe penetration. The integration of catalyzed reporter deposition (CARD-FISH) has significantly improved detection sensitivity for microorganisms with low ribosomal RNA content, while the emergence of CRISPR-based FISH methods (CRISPR-FISH) offers enhanced signal-to-noise ratios and greater design flexibility [7].
For microbial ecology studies, FISH provides unparalleled insights into the spatial organization of microbial communities, enabling researchers to investigate microbial interactions, niche specialization, and community dynamics in situ. When combined with advanced imaging techniques such as confocal laser scanning microscopy and super-resolution microscopy, FISH can reveal the intricate spatial relationships between different microbial taxa and their environment at unprecedented resolution.
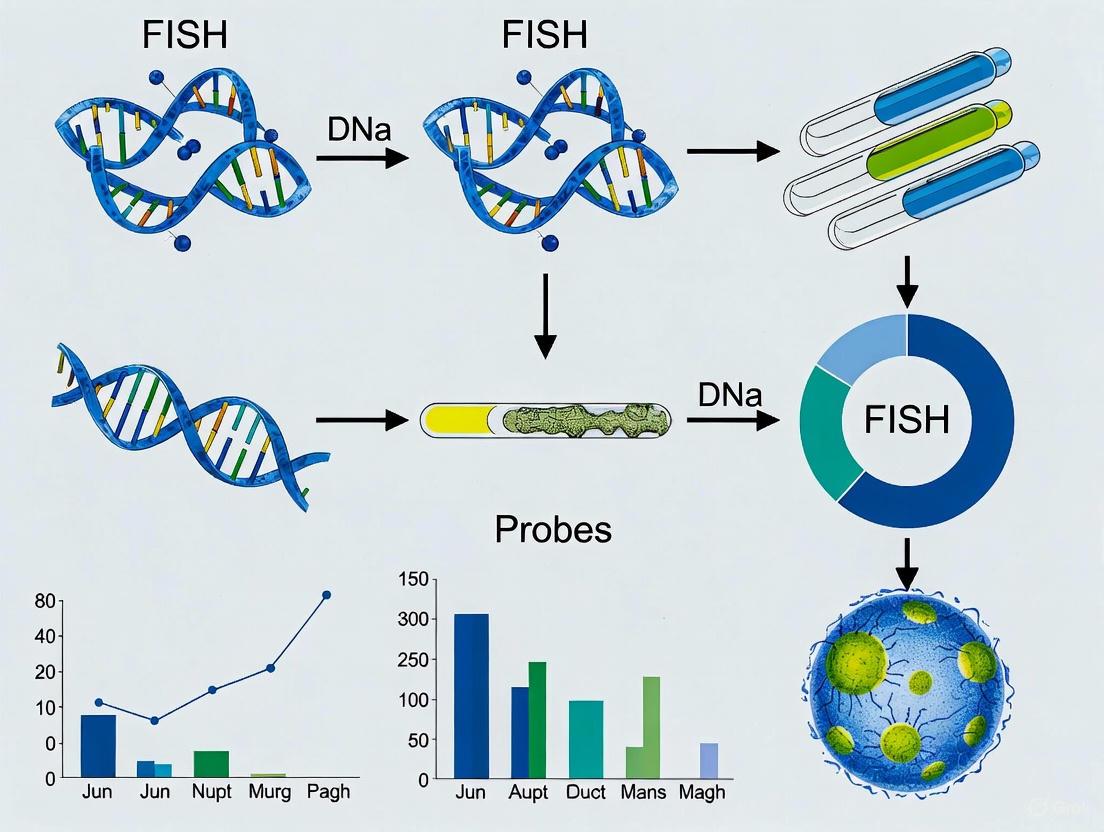
Diagram 1: Microbial FISH Workflow
Advanced FISH Protocols and Methodologies
Live-FISH for Soil Microbiomes
The Live-FISH protocol represents a significant advancement for targeting viable microorganisms in complex environmental samples. The following protocol has been adapted from a 2025 study evaluating Live-FISH applicability on soil microbiomes [5]:
Materials and Reagents:
- Fresh soil samples (temperate topsoil used in original study)
- Phosphate-buffered saline (PBS), pH 7.4
- Paraformaldehyde solution (4% in PBS)
- Ethanol series (50%, 80%, 96%)
- Hybridization buffer (0.9 M NaCl, 20 mM Tris/HCl, 0.01% SDS, XX% formamide - concentration optimized for specific probe)
- Cy3-labeled rRNA-targeted oligonucleotide probes
- Washing buffer (20 mM Tris/HCl, 0.01% SDS, XX mM NaCl - concentration matching formamide in hybridization buffer)
- Low-melting-point agarose (0.1% final concentration)
- Fluorescence-activated cell sorting (FACS) system
Methodology:
- Sample Preparation: Homogenize 1 g fresh soil in 10 mL PBS. Remove large particles by low-speed centrifugation (500 × g, 2 min).
- Cell Fixation: Fix cells in suspension with paraformaldehyde (4% final concentration) for 2-4 hours at 4°C.
- Permeabilization: Apply ethanol series (50%, 80%, 96%) for 3 minutes each to enhance cell wall permeability.
- Hybridization: Mix 8 μL cell suspension with 2 μL hybridization buffer containing Cy3-labeled probe (50 ng/μL final concentration). Incubate for 2-3 hours at appropriate hybridization temperature (varies based on probe sequence and formamide concentration).
- Stringency Washes: Incubate samples in pre-warmed washing buffer for 10-15 minutes at hybridization temperature.
- Cell Embedding: Resuspend cells in 0.1% low-melting-point agarose for stabilization during microscopy and sorting.
- Viability Assessment: Apply propidium monoazide (PMA) treatment to differentiate between viable and non-viable cells.
- Microscopy and Cell Sorting: Analyze samples by epifluorescence microscopy or sort labeled cells using FACS for cultivation attempts.
Critical Considerations:
- Taxon-specific effects necessitate protocol optimization for different microbial groups
- Planctomycetota and Bacillota have demonstrated better viability retention than Acidobacteriota [5]
- Hybridization conditions (temperature, formamide concentration) must be optimized for each probe
- PMA treatment is essential for accurate viability assessment
U-FISH: AI-Assisted Signal Detection
The recent development of U-FISH represents a significant advancement in FISH image analysis, addressing the challenge of accurate signal spot detection across diverse imaging conditions [9]. This deep learning method transforms raw FISH images with variable characteristics into enhanced images with uniform signal spots and improved signal-to-noise ratio.
Implementation Protocol:
- Image Acquisition: Collect FISH images according to standard protocols for your specific application.
- Software Installation: Download U-FISH package from available repositories (compatible with Python 3.8+).
- Model Application: Process images through the pre-trained U-Net model (163k parameters) for universal spot detection.
- Parameter Optimization: Utilize fixed parameters across all datasets without need for manual adjustment.
- Analysis: Employ integrated quantification tools for signal counting and localization.
Performance Characteristics:
- Superior accuracy (F1 score: 0.924) compared to existing methods (deepBlink: 0.901, DetNet: 0.886) [9]
- Enhanced generalizability across diverse FISH datasets
- Compatibility with 3D FISH data analysis
- Integration with large language models for simplified user interaction
Essential Research Reagent Solutions
Successful implementation of FISH protocols requires specific reagent systems optimized for different applications and sample types. The following table outlines key reagent solutions essential for modern FISH applications in microbial detection research.
Table 3: Essential Research Reagent Solutions for FISH Applications
| Reagent Category | Specific Examples | Function | Application Notes |
|---|---|---|---|
| Fixation Reagents | Paraformaldehyde (4%), Ethanol:Acetic Acid (3:1) | Preserve cellular morphology and nucleic acid integrity | Paraformaldehyde preferred for microbial cells; optimal fixation time varies by cell type |
| Permeabilization Agents | Triton X-100, Lysozyme, Proteinase K | Enhance probe accessibility to target sequences | Concentration and duration must be optimized to balance signal and morphology preservation |
| Hybridization Buffers | Standard saline citrate (SSC) with formamide | Create optimal stringency conditions for specific hybridization | Formamide concentration determines stringency; must match probe design characteristics |
| Fluorescent Labels | Cy3, Cy5, FITC, Quantum dots | Provide detection signal through fluorescence emission | Quantum dots offer superior photostability for multiplex applications [6] |
| Signal Amplification Systems | Tyramide signal amplification (TSA), HCR | Enhance detection sensitivity for low-abundance targets | Essential for microorganisms with low rRNA content; may increase background |
| Mounting Media | Antifade reagents (Vectashield, ProLong) | Reduce photobleaching during microscopy | Choice affects signal longevity and compatibility with super-resolution techniques |
| Cell Viability Markers | Propidium monoazide (PMA), SYTO dyes | Differentiate between viable and non-viable cells | Critical for Live-FISH applications and accurate interpretation of results [5] |
Future Perspectives and Emerging Applications
The future trajectory of FISH technology points toward several promising directions that will further expand its applications in microbial detection research. The integration of artificial intelligence and machine learning into image analysis workflows represents one of the most significant advancements, with tools like U-FISH demonstrating superior accuracy and generalizability across diverse datasets [9]. These AI-assisted platforms are increasingly being integrated with large language models to create more intuitive user interfaces, making sophisticated analysis accessible to researchers without specialized computational backgrounds.
The continued development of multiplexing capabilities will enable simultaneous detection of dozens or even hundreds of microbial taxa within a single sample, providing unprecedented insights into community structure and spatial organization. Emerging technologies such as CRISPR-based FISH methods (CRISPR-FISH, CRISPR-Hyb) offer promising alternatives with potentially higher signal-to-noise ratios and greater design flexibility [7]. These approaches may overcome current limitations in probe penetration and hybridization efficiency, particularly for environmental microorganisms with robust cell walls.
The application of FISH in spatial-omics represents another frontier, with techniques increasingly being integrated with other molecular approaches to provide multi-omics data within morphological context. The combination of FISH with immunofluorescence (FISH-IF) allows simultaneous visualization of nucleic acids and proteins, while the correlation of FISH data with metagenomic information provides deeper functional insights [8]. These integrated approaches are particularly valuable for understanding the functional capabilities of uncultivated microorganisms in complex environments.
From a technological perspective, the miniaturization and automation of FISH protocols will facilitate higher throughput applications and more standardized results across laboratories. Automated imaging platforms coupled with sophisticated analysis software are already reducing inter-observer variability and accelerating processing times, making FISH more accessible for routine monitoring and clinical diagnostics [7]. As these technologies continue to evolve, FISH will likely become an increasingly integral component of comprehensive microbial characterization workflows across research, clinical, and industrial applications.
Diagram 2: FISH Technology Future Directions
Fluorescence in situ hybridization (FISH) is a powerful molecular cytogenetic technique that uses fluorescent probes to bind specific nucleic acid sequences with high complementarity, allowing for the visualization and identification of microorganisms within their environmental context [1]. The development of FISH has revolutionized microbial ecology by enabling researchers to detect and quantify microorganisms, study their spatial distribution in complex environments like biofilms, and investigate their ecological functions, all without the need for cultivation [11] [12]. The efficacy of FISH technology heavily depends on probe design, which must achieve a delicate balance between sensitivity to target sequences and specificity to avoid cross-hybridizations with unrelated sequences [13].
The evolution of FISH probes has progressed from initial DNA and RNA probes to advanced Nucleic Acid Mimics (NAMs), including peptide nucleic acids (PNA) and locked nucleic acids (LNA), which offer enhanced sensitivity, specificity, and resistance to enzymatic degradation [11] [14]. These advancements have expanded FISH applications from merely identifying microbial community composition using high-copy rRNA targets to detecting specific functional genes, mRNA transcripts, and even single-copy genes with improved signal amplification techniques [12]. This document provides detailed application notes and protocols for the major FISH probe types, framed within the context of microbial detection research for scientists and drug development professionals.
Fundamental Probe Types: DNA and rRNA-Targeting Probes
DNA Probes
DNA probes are single-stranded fragments of DNA, typically ranging from 20 to 50 nucleotides, that are complementary to a nucleotide sequence of interest [1]. These probes can be generated through various methods, including nick translation or polymerase chain reaction (PCR) using tagged nucleotides, and are typically labelled with fluorophores directly or with targets for antibodies or biotin for indirect detection [1]. For microbial detection, DNA probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in genome projects, with artificial chromosomes (such as BAC) serving as common sources [1].
The specificity of DNA probes varies with their design. Whole-chromosome painting probes hybridize along an entire chromosome and are used to count chromosomes, show translocations, or identify extra-chromosomal chromatin fragments [1]. Locus-specific probe mixtures target specific regions of DNA and are particularly useful for detecting deletion mutations or specific translocations [1]. In comparative studies, commercially produced digoxigenin-labelled DNA probes have demonstrated effectiveness in detecting various DNA viruses, including canine bocavirus 2 (CBoV-2) and porcine circovirus 2 (PCV-2) in infected tissues [15].
rRNA-Targeting Probes
Ribosomal RNA (rRNA) has traditionally been the primary target for FISH in microbial detection due to several advantageous characteristics [12]. rRNA is present in all living cells in relatively high copy numbers (10â´â€“10âµ in an actively growing cell), providing abundant natural amplification for detection [12]. Furthermore, as a traditional phylogenetic marker, extensive rRNA sequence databases are available for probe design across diverse taxonomic groups [12].
The procedure for rRNA-targeted FISH typically involves sample fixation (often with paraformaldehyde) to preserve cellular structure and permeabilization to facilitate probe access to the target rRNA [12]. Hybridization is then performed under optimized conditions of temperature, pH, and salt concentration, followed by washing steps to remove unbound probes and visualization via fluorescence microscopy [11]. The accessibility of target sites on rRNA can vary significantly due to secondary and tertiary structures and ribosomal protein interactions, which presents a challenge for consistent hybridization efficiency [16].
Table 1: Comparison of Fundamental FISH Probe Types for Microbial Detection
| Probe Characteristic | DNA Probes | rRNA-Targeting Probes |
|---|---|---|
| Target Molecule | DNA sequences (genomic DNA, genes, plasmids) | Ribosomal RNA (16S or 23S rRNA) |
| Copy Number per Cell | Varies (single copy genes to multiple copies) | High (10â´â€“10âµ in active cells) |
| Primary Applications | Gene detection, chromosomal painting, translocation studies | Phylogenetic identification, microbial community analysis |
| Detection Sensitivity | Lower for single-copy genes; requires amplification | High due to natural amplification from abundant rRNA |
| Design Considerations | Specificity to target sequence; probe length (20-50 nt) | Target accessibility; phylogenetic specificity |
| Limitations | Low signal for low-copy targets; requires signal amplification | Dependent on cellular activity and ribosome content |
Advanced Nucleic Acid Mimics (PNA and LNA)
Peptide Nucleic Acid (PNA) Probes
Peptide Nucleic Acids (PNA) represent a significant advancement in FISH probe technology, featuring a neutral pseudopeptide backbone that replaces the sugar-phosphate backbone of natural nucleic acids [14]. This structural modification confers several advantageous properties: higher thermal stability of PNA-DNA/PNA-RNA duplexes, resistance to enzymatic degradation by nucleases and proteases, and flexibility in target selection due to their ability to bind to complementary sequences with high affinity and specificity [11] [14]. The neutral backbone reduces electrostatic repulsion with target nucleic acids, allowing PNA probes to hybridize under lower salt conditions that would destabilize DNA-DNA or DNA-RNA duplexes, which is particularly beneficial for penetrating through the complex cell walls of microorganisms [14].
PNAs have demonstrated particular utility in clinical diagnostics for the rapid identification of bacterial pathogens directly from blood cultures, including Staphylococcus aureus [12]. Their enhanced penetration characteristics make them valuable for detecting microorganisms with tough cell walls that are difficult to permeabilize with standard FISH protocols [14]. Furthermore, PNA-FISH applications have been successfully implemented for the identification of indicator microorganisms using standardized methods, facilitating more efficient monitoring of microbial contamination [12].
Locked Nucleic Acid (LNA) Probes
Locked Nucleic Acids (LNA) are another class of nucleic acid mimics characterized by a bicyclic sugar ring where a 2'-O,4'-C methylene bridge "locks" the ribose moiety in a C3'-endo conformation [14]. This locked structure enhances base stacking and backbone pre-organization, resulting in unprecedented hybridization affinity toward complementary DNA and RNA sequences [14]. LNA probes demonstrate superior mismatch discrimination capabilities compared to DNA probes, making them exceptionally valuable for applications requiring single-nucleotide polymorphism (SNP) discrimination [14].
A significant advantage of LNA technology is its compatibility with standard DNA synthesis methods, allowing for the creation of LNA-DNA mixmers (chimeric oligonucleotides containing both LNA and DNA monomers) [14]. This flexibility enables precise optimization of the thermal stability and specificity of FISH probes by strategically incorporating LNA monomers at critical positions within the probe sequence [14]. The high binding affinity of LNA probes allows for the use of shorter probe sequences while maintaining specificity, which can be advantageous for accessing structurally constrained target sites [14].
Table 2: Performance Comparison of Nucleic Acid Mimics in FISH Applications
| Performance Metric | PNA Probes | LNA Probes | Traditional DNA Probes |
|---|---|---|---|
| Binding Affinity | High | Very High | Moderate |
| Nuclease Resistance | Excellent | Good | Poor |
| Cell Penetration | Excellent | Moderate | Variable (requires permeabilization) |
| Sequence Specificity | High | Very High (excellent mismatch discrimination) | Moderate |
| Hybridization Conditions | Low salt conditions | Standard FISH conditions | Standard FISH conditions |
| Design Flexibility | Compatible with standard DNA synthesis | Compatible with standard DNA synthesis | High flexibility |
| Best Applications | Tough cell walls, clinical diagnostics | SNP detection, structured targets | General purpose, multiplexing |
Comparative Analysis and Performance Data
The performance of different FISH probe types has been systematically evaluated across various applications and experimental conditions. A comprehensive 2018 study comparing ISH techniques for virus detection found that the detection rate and cell-associated positive area were highest using a commercially available FISH-RNA probe mix compared to self-designed digoxigenin-labelled RNA probes or commercially produced digoxigenin-labelled DNA probes [15]. This superior performance was attributed to the multiple amplification steps and specialized probe design inherent to the FISH-RNA system.
The thermodynamic properties of probe hybridization significantly influence FISH efficiency. Research has demonstrated that the overall Gibbs free energy change (ΔG°overall) is a strong predictor of hybridization efficiency, superior to conventional estimates based on the dissociation temperature of DNA/rRNA duplexes [16]. A threshold ΔG°overall of -13.0 kcal/mol has been proposed as a goal in FISH probe design to maximize hybridization efficiency without compromising specificity [16]. This mechanistic approach considers not only the DNA-RNA hybridization but also intramolecular DNA and rRNA interactions that occur during FISH, providing a more comprehensive understanding of probe affinity [16].
Environmental factors including temperature, buffer composition, salt concentration, and hybridization time critically influence the efficiency and specificity of FISH across all probe types [13]. In standard FISH protocols, hybridization typically occurs at 46°C for 2-3 hours with salt concentration maintained at 750 mM NaCl and 87.5 mM sodium citrate [13]. However, optimal hybridization conditions should be determined individually for each FISH assay, as higher temperatures may increase hybridization rate but risk non-specific binding, while lower temperatures may enhance specificity but reduce hybridization rate [13].
Table 3: Experimental Protocol Parameters for Different FISH Probe Types
| Protocol Parameter | DNA Probes | rRNA Probes | PNA Probes | LNA Probes |
|---|---|---|---|---|
| Typical Probe Length | 20-50 nucleotides | 15-25 nucleotides | 15-20 nucleotides | 15-25 nucleotides |
| Hybridization Temperature | 46°C | 46°C | 55-65°C | Variable (depends on LNA content) |
| Formamide in Hybridization Buffer | 0-50% | 0-50% | Often omitted | 0-50% |
| Hybridization Time | 2-3 hours | 2-3 hours | 30-90 minutes | 2-3 hours |
| Salt Concentration | 750 mM NaCl, 87.5 mM sodium citrate | 750 mM NaCl, 87.5 mM sodium citrate | Lower salt conditions | 750 mM NaCl, 87.5 mM sodium citrate |
| Wash Temperature | 48°C | 48°C | 55-65°C | Variable (depends on LNA content) |
| Permeabilization Requirement | High | High | Reduced | Moderate to High |
Detailed Experimental Protocols
Standard FISH Protocol for rRNA-Targeted Detection
The following protocol describes the standard procedure for FISH using rRNA-targeted DNA probes for microbial detection in environmental samples:
Sample Fixation: Fix cells or tissue sections with appropriate fixatives (commonly 4% formaldehyde or paraformaldehyde in phosphate-buffered saline) for 1-3 hours at room temperature to preserve cellular structure and nucleic acid integrity [11] [1]. For FFPET (formalin-fixed paraffin-embedded tissues), sections of 2-3 µm thickness are typically used [15].
Permeabilization: Treat fixed samples with detergents at 0.1% concentration (Tween-20 or Triton X-100) to enhance tissue permeability and allow probe penetration [11] [1]. For difficult-to-lyse cells, additional enzymatic treatments (lysozyme, proteinase K) may be required [12].
Probe Denaturation: Denature probe mixtures at 85-90°C for 5-10 minutes immediately before use, then place on ice to prevent reannealing [11].
Hybridization: Apply target-specific probe in hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl pH 7.4, 0.01% SDS, 35% formamide) and incubate for 2-3 hours at 46°C [13] [17]. The optimal formamide concentration should be determined empirically for each probe.
Washing: Perform stringent washes with pre-warmed wash buffer (20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS, 0.080 M NaCl) at 48°C for 20 minutes to remove nonspecific hybrids and unbound probe molecules [11] [17]. Ethanol washes may be included to reduce autofluorescence [11].
Counterstaining and Visualization: Apply appropriate counterstains (DAPI for DNA, etc.) and mount samples for fluorescence microscopy examination using confocal fluorescence microscopy or wide-field epifluorescence systems [11] [1].
Figure 1: Standard FISH Workflow for Microbial Detection
Live-FISH Protocol for Viable Cell Detection
The live-FISH protocol enables specific hybridization in living bacterial cells, allowing for subsequent cultivation and functional studies:
Sample Preparation: Grow bacterial cultures to late logarithmic growth phase (OD₆₀₀ₙₘ = 0.5-0.8) in appropriate medium. Concentrate environmental samples by filtration through 0.2 µm filters if necessary [17].
Washing: Wash cells three times with 1x Phosphate Buffered Saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Naâ‚‚HPOâ‚„, 1.8 mM KHâ‚‚POâ‚„) or artificial seawater (ASW) to remove growth medium, avoiding ethanol treatments that would kill cells [17].
Transformation: Resuspend washed cells in 50 µl of 100 mM CaCl₂, then incubate for 15 minutes on ice with 4 ng/µl of fluorescent probe. Perform heat shock at 42°C for 60 seconds, then return briefly to ice [17].
Hybridization: Immediately add 500 µl of pre-warmed (46°C) hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl pH 7.4, 0.01% SDS, 35% formamide) and incubate for 2 hours at 46°C with shaking at 200 rpm [17].
Washing: Pellet cells at 10,000 × g for 5 minutes and resuspend in 500 µl of pre-warmed (48°C) wash buffer (20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS, 0.080 M NaCl). Incubate at 48°C for 20 minutes, then centrifuge twice in 500 µl of ice-cold 1x PBS [17].
Cell Sorting and Cultivation: Keep cells in PBS buffer on ice until sorting using fluorescence-activated cell sorting (FACS). Sort specific taxonomic groups of bacteria and subsequently culture them on appropriate non-selective media [17].
Research Reagent Solutions
Table 4: Essential Research Reagents for FISH Experiments
| Reagent/Category | Specific Examples | Function/Purpose |
|---|---|---|
| Fixatives | 4% Formaldehyde, Paraformaldehyde (PFA) | Preserves cellular structure and nucleic acid integrity |
| Permeabilization Agents | Tween-20, Triton X-100 (0.1%), Lysozyme, Proteinase K | Enhances cell wall/membrane permeability for probe entry |
| Hybridization Buffers | 0.9 M NaCl, 20 mM Tris-HCl pH 7.4, 0.01% SDS, 35% Formamide | Creates optimal conditions for specific probe-target hybridization |
| Wash Buffers | 20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS, 0.080 M NaCl | Removes nonspecific hybrids and unbound probes |
| Probe Types | DNA probes, RNA probes, PNA probes, LNA probes | Targets specific nucleic acid sequences for detection |
| Fluorophores | 6-FAM, Cy3, FITC, Cy5 | Provides detectable signal for microscopy |
| Signal Amplification Systems | Tyramide Signal Amplification (TSA), Multiple Labeled Probes | Enhances detection sensitivity for low-abundance targets |
| Commercial Probe Suppliers | Abbott Laboratories, Agilent Technologies, Thermo Fisher Scientific, F. Hoffmann-La Roche Ltd. | Sources of validated probes and FISH reagents |
Advanced Applications and Future Perspectives
The development of advanced FISH probe types has enabled increasingly sophisticated applications in microbial detection and research. Single-molecule RNA FISH (smFISH or Stellaris RNA FISH) allows for the detection and quantification of individual mRNA molecules in tissue samples through the application of multiple short singly labeled oligonucleotide probes (up to 48 probes targeting a single mRNA molecule) [1]. This technology provides sufficient localized fluorescence to accurately detect and localize each target mRNA, with applications in cancer diagnosis, neuroscience, gene expression analysis, and companion diagnostics [1].
Multiplexed identification techniques such as Combinatorial Labeling and Spectral Imaging FISH (CLASI-FISH) enable simultaneous analysis of multiple microbial taxa by combining combinatorial labeling with spectral imaging to distinguish numerous microbes simultaneously through linear unmixing of fluorophore spectra [11]. While CLASI-FISH offers powerful multiplexing capabilities, it may suffer from internal sensitivity loss and potential probe binding bias, limitations that alternative approaches like Double Labeling of Oligonucleotide Probes for FISH (DOPE-FISH) attempt to address through double signal intensity and stable specificity [11].
The future of FISH probe development continues to evolve with emerging technologies. Expansion-Assisted Iterative (EASI)-FISH has been developed for examining the 3D organization of cell types in thick tissues, particularly valuable for characterizing complex architectures like brain function [11]. Resolution After Single-strand Exonuclease Resection (RASER)-FISH provides robust generation of single-stranded DNA with excellent preservation of chromatin structure and nuclear integrity through exonuclease digestion rather than physical denaturation, resulting in improved hybridization efficiency [11]. These advancements, combined with the ongoing refinement of NAM chemistry and signal amplification strategies, promise to further expand the capabilities of FISH for microbial detection and characterization in complex environments.
Figure 2: FISH Probe Types and Their Primary Applications
Fluorescence in situ hybridization (FISH) with ribosomal RNA (rRNA) targeting has become a cornerstone technique in microbial ecology and diagnostics. This method provides a powerful approach for the cultivation-independent identification, visualization, and quantification of microorganisms in their natural environments [11] [18]. The technique hinges on the use of fluorescently labeled oligonucleotide probes that bind to complementary rRNA sequences within microbial cells, allowing researchers to detect specific taxa while preserving their spatial context within complex samples like biofilms, tissues, and environmental samples [11] [19]. The selection of an appropriate molecular target is paramount to the success of any FISH experiment, and among available options, ribosomal RNA offers a unique combination of characteristics that make it exceptionally suitable for microbial detection and identification. This application note details the theoretical and practical framework underlying the targeted detection of microorganisms via rRNA-FISH, providing structured data, validated protocols, and key resources to facilitate implementation in research and diagnostic settings.
The Molecular Basis for Targeting rRNA
Ribosomal RNA molecules, particularly the 16S rRNA in prokaryotes and 18S rRNA in eukaryotes, serve as ideal targets for FISH due to their universal distribution, high cellular abundance, and conserved yet variable sequence regions. The rRNA genes are present in all living cells, and the transcribed rRNA molecules can number in the thousands to tens of thousands per cell, providing an naturally amplified target that facilitates sensitive detection without the need for signal amplification in many applications [11] [20]. Furthermore, the genetic sequences of rRNAs contain a mixture of highly conserved regions and variable domains. The conserved areas enable the design of broad-range probes targeting entire domains (e.g., bacteria or archaea), while the variable regions allow for the design of probes with specificity at various taxonomic levels, from genus to species [20] [21].
The advent of comprehensive rRNA databases, such as the Comparative RNA Web (CRW) Site and the Ribosomal Database Project (RDP), has been instrumental in advancing rRNA-targeted FISH [20] [21]. These resources provide extensive collections of aligned rRNA sequences that are crucial for in silico probe design and validation. Probe design software can leverage these databases to identify unique target sequences and check for potential cross-hybridization with non-target organisms, thereby maximizing probe specificity and coverage [20].
Table 1: Key Advantages of Ribosomal RNA as a FISH Target
| Feature | Description | Application in FISH |
|---|---|---|
| High Cellular Abundance | rRNAs can constitute up to 80% of the total cellular RNA, with copy numbers ranging from (10^3) to (10^5) per cell [11]. | Provides a naturally amplified signal, enabling detection without enzymatic amplification and facilitating high sensitivity. |
| Universal Distribution | rRNA genes and their products are found in all living cells, from bacteria and archaea to eukaryotes [21]. | Allows for the development of universal detection assays and the study of diverse, complex microbial communities. |
| Evolutionary Conservation | Contains stretches of sequence that are highly conserved across broad phylogenetic groups [20] [21]. | Enables design of broad-range probes (e.g., for all Bacteria or Archaea) to assess total microbial load or community structure. |
| Variable Sequence Regions | Interspersed with regions of sequence variation that are phylogenetically informative [20] [22]. | Allows for design of group-, genus-, or species-specific probes for taxonomic identification and quantification. |
| Well-Established Databases | Large, curated databases of rRNA sequences (e.g., SILVA, RDP, CRW) are publicly available [20] [21]. | Facilitates systematic, computer-aided design and validation of probes for specificity and coverage before experimental use. |
Quantitative Analysis of Probe Efficacy
The performance of rRNA-targeted FISH probes is governed by thermodynamic principles that can be modeled to predict hybridization behavior. Key parameters include probe specificity, which is the ability to uniquely hybridize to the target group, and probe coverage, the proportion of organisms within the target group that possess the exact probe sequence [20]. A systematic analysis of published probes revealed that many have insufficient coverage or specificity for their intended target group when re-evaluated against modern, expanded rRNA databases [20].
Thermodynamic models, such as those implemented in the mathFISH software, help predict the formamide melting profile of a probe—the concentration of formamide at which the probe dissociates from its target. This is critical for establishing stringent hybridization conditions that maximize specific binding while minimizing non-specific signal [20]. The use of unlabeled competitor oligonucleotides is a common strategy to block hybridization to non-targets with slightly mismatched sequences, thereby improving effective specificity [20]. Furthermore, requiring the simultaneous hybridization of two independent probes for positive identification dramatically increases specificity. Research has demonstrated that while highly specific probes can be designed for only about a third of bacterial genera using a single probe, this proportion rises to over two-thirds when two-probe sets are employed [20].
Table 2: Strategies for Enhancing Specificity in rRNA-Targeted FISH
| Strategy | Mechanism | Impact on Specificity |
|---|---|---|
| Stringency Control | Manipulation of formamide concentration in the hybridization buffer to control nucleic acid denaturation [20]. | Higher formamide concentrations destabilize mismatched hybrids, suppressing false positives from non-targets. |
| Competitor Probes | Use of unlabeled oligonucleotides that bind to near-perfect matching non-target sequences, blocking probe access [20]. | Diminishes or eliminates signal from non-target organisms, thereby improving the signal-to-noise ratio. |
| Multiple Probe Hybridization | Requiring positive signal from two or more independently targeting probes for a positive identification [20]. | Significantly reduces false positives, as the probability of non-specific binding of multiple probes to the same cell is low. |
| Error-Robust Encoding | Using sequential FISH with barcoding schemes that tolerate and correct for single-bit hybridization errors [19]. | Enables highly multiplexed imaging while maintaining high identification accuracy within complex communities. |
Core Protocol: rRNA-Targeted FISH for Microbial Detection
The following protocol provides a standardized workflow for detecting microorganisms using rRNA-targeted FISH. This protocol is adapted from established methods and can be applied to a variety of sample types, including pure cultures, environmental samples, and clinical specimens [11] [20] [18].
Sample Fixation and Permeabilization
Objective: To preserve cellular morphology and integrity while allowing probe penetration. Procedure:
- Harvesting: Collect microbial cells by centrifugation (e.g., 10,000 × g for 5 min) for liquid cultures or by scraping for surface-grown biofilms.
- Washing: Gently resuspend the cell pellet in 1× phosphate-buffered saline (PBS) and repeat centrifugation.
- Fixation: Resuspend the cell pellet in a fixation solution. For most Gram-negative bacteria, use 3% (v/v) paraformaldehyde (PFA) in PBS for 1-4 hours at room temperature or overnight at 4°C. For Gram-positive bacteria, an additional step of 50% ethanol in PBS may be used for improved permeabilization [18].
- Storage: After fixation, pellet the cells, wash with PBS, and finally resuspend in a 1:1 mixture of PBS and ethanol for storage at -20°C. Fixed samples can be stored for several months.
Probe Hybridization
Objective: To facilitate the specific binding of fluorescently labeled oligonucleotide probes to target rRNA sequences. Reagents:
- Hybridization Buffer: Typically contains a high-salt concentration (e.g., 0.9 M NaCl), formamide (concentration must be optimized for each probe), Tris-HCl (pH ~8.0), and a detergent like SDS [20] [18].
- Labeled Probe(s): Oligonucleotide probe(s) (typically 15-25 nucleotides) labeled at the 5'-end with a fluorophore (e.g., Cy3, Cy5, FLUOS, or derivatives). Probes are used at a final concentration of 1-5 µg/mL. Procedure:
- Preparation: Spot fixed samples onto glass slides and allow to air dry.
- Dehydration: Dehydrate the sample by successive immersion in 50%, 80%, and 96% ethanol (3 min each).
- Hybridization: Apply an appropriate volume of hybridization buffer containing the probe(s) to the sample spot and cover with a coverslip to prevent evaporation.
- Incubation: Incubate the slide in a dark, humidified chamber at the appropriate hybridization temperature (typically 46°C) for 1.5 to 3 hours, or overnight.
Post-Hybridization Washing and Visualization
Objective: To remove unbound and non-specifically bound probes, reducing background fluorescence. Reagents: - Wash Buffer: Similar to hybridization buffer but with a lower salt concentration (e.g., 80 mM NaCl) and the same concentration of formamide. Pre-warm before use. Procedure: 1. Washing: Carefully remove the coverslip and immerse the slide in pre-warmed wash buffer. Incubate at 48°C for 10-20 minutes. 2. Rinsing: Briefly rinse the slide with cold, distilled water to remove salt crystals and allow to air dry in the dark. 3. Mounting: Apply an anti-fading mounting medium (e.g., Vectashield or Citifluor) and a coverslip. 4. Visualization: Observe the sample under an epifluorescence or confocal laser scanning microscope equipped with appropriate filter sets for the fluorophore(s) used.
Diagram 1: Core FISH Workflow
Advanced Multiplexing with Sequential FISH
For complex microbial communities, identifying numerous taxa simultaneously is crucial. Sequential error-robust FISH (SEER-FISH) significantly increases multiplexing capability by using multiple rounds of probe hybridization, imaging, and probe dissociation [19]. In each round, a subset of taxa is labeled with one of F fluorophores. Over N rounds, this generates a unique N-bit barcode for each taxon, allowing for the theoretical identification of F^N different microbes [19]. Error-robust encoding schemes with a defined minimal Hamming distance (e.g., HD=4) between barcodes allow for the correction of detection errors that may occur during sequential hybridization, ensuring high accuracy in taxonomic identification [19].
Diagram 2: SEER-FISH Multiplexing
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Reagents for rRNA-Targeted FISH Experiments
| Reagent / Material | Function | Example / Note |
|---|---|---|
| Paraformaldehyde (PFA) | Cross-linking fixative that preserves cellular structure and immobilizes nucleic acids. | Typically used at 2-4% in PBS. Handle in a fume hood. |
| Ethanol | Dehydrating agent and permeabilization aid; also used for sample storage. | A graded ethanol series (50%, 80%, 96%) is used for dehydration. |
| Formamide | Denaturing agent used to control stringency in hybridization and wash buffers. | Concentration is probe-specific; higher % increases stringency. |
| Oligonucleotide Probes | Fluorescently labeled DNA strands complementary to target rRNA sequences. | Designed for specificity; often 15-25 nt long, labeled with Cy3, Cy5, FAM, etc. |
| Competitor Oligonucleotides | Unlabeled probes used to block non-specific binding to off-target sequences. | Designed to bind near-perfect matches in non-target organisms [20]. |
| Hybridization & Wash Buffers | Provide ionic strength and pH for controlled nucleic acid hybridization and washing. | Contain salts (NaCl), buffer (Tris/HCl), detergent (SDS), and formamide. |
| Anti-fading Mountant | Preserves fluorescence and reduces photobleaching during microscopy. | Commercial products like Vectashield or Citifluor are commonly used. |
| Einecs 235-359-4 | Samarium Cobalt (SmCo3)|EINECS 235-359-4 | Samarium Cobalt (SmCo3), EINECS 235-359-4, is a high-performance magnetic intermetallic compound for research applications. For Research Use Only. Not for human use. |
| alpha-L-fructofuranose | alpha-L-fructofuranose|Research Use Only |
Ribosomal RNA remains the preeminent target for FISH-based microbial detection due to its unique combination of high cellular abundance, phylogenetic relevance, and the robust framework of databases and thermodynamic models available for probe design. The protocols and strategies outlined here, from basic FISH to advanced multiplexing with SEER-FISH, provide researchers with a powerful set of tools to visualize and identify microorganisms in situ. As sequencing technologies continue to expand our knowledge of microbial diversity, and as microscopy and probe chemistries advance, the utility and application of rRNA-targeted FISH will continue to grow, solidifying its role as an indispensable technique in microbial ecology, diagnostics, and therapeutic development.
Fluorescence *in situ hybridization (FISH) represents a cornerstone molecular cytogenetic technique that enables the detection and localization of specific DNA sequences on chromosomes within cells [23]. Since its inception, FISH technology has diversified significantly, giving rise to numerous advanced variants that address key limitations in sensitivity, multiplexing capacity, and specificity. This technological evolution has been particularly impactful in microbial detection research, where the ability to identify, quantify, and spatially localize microorganisms within complex environmental or clinical samples provides crucial insights into community structure, function, and dynamics.
The expansion of FISH methodologies has transformed microbial ecology and diagnostics, moving beyond simple identification to encompass functional analysis, gene expression monitoring, and intricate spatial profiling within biofilms, tissues, and environmental samples. This overview focuses on three significant FISH variants—CARD-FISH, CLASI-FISH, and DOPE-FISH—each representing distinct technological advancements that address specific research challenges in microbial detection and visualization.
Key FISH Variants: Principles and Applications
Table 1: Comparative Overview of Advanced FISH Variants
| Technique | Full Name | Key Feature | Primary Application in Microbial Research | Sensitivity/Signal Amplification | Multiplexing Capacity |
|---|---|---|---|---|---|
| CARD-FISH | Catalyzed Reporter Deposition FISH | Enzyme-mediated tyramide signal amplification | Detection of low-abundance targets; quantitative gene expression analysis [24] [25] | Very high (10-20x increase vs monolabeled probes) [26] | Low to moderate |
| DOPE-FISH | Double Labeling of Oligonucleotide Probes FISH | Dual fluorophore labeling on single oligonucleotide | Increased sensitivity for rare targets; simultaneous multicolor detection [26] | ~2x increase vs mono-labeled probes [26] | Moderate |
| CLASI-FISH | Combinatorial Labeling and Spectral Imaging FISH | Combinatorial probe labeling with spectral imaging | High-phylogenetic diversity community analysis; spatial mapping of complex microbiomes [19] | Standard | Very high (theoretically 2F-1 targets with F fluorophores) [19] |
CARD-FISH (Catalyzed Reporter Deposition FISH)
CARD-FISH addresses a fundamental limitation in conventional FISH: detecting targets with low cellular abundance. This method replaces the fluorescently-labeled probe used in standard FISH with an oligonucleotide conjugated to horseradish peroxidase (HRP) [26]. After the probe hybridizes to its target sequence, the HRP enzyme catalyzes the deposition of multiple labeled tyramide molecules at the site of hybridization. This signal amplification system generates a much stronger fluorescence signal than directly-labeled probes, enabling detection of target sequences that would otherwise remain undetectable.
The exceptional sensitivity of CARD-FISH, which provides a 10-20 fold increase in signal intensity compared to standard monolabeled probes, makes it particularly valuable for environmental microbiology [26]. It has been successfully applied to investigate gene expression heterogeneity in cyanobacteria at the single-cell level, providing insights into physiological processes within populations of filamentous Trichodesmium and single-celled Synechocystis and Cyanothece [24]. Furthermore, CARD-FISH has been instrumental in protistan ecology, helping to uncover the in situ abundance, feeding modes, and grazing preferences of diverse nanoplanktonic flagellate lineages in aquatic environments [25].
DOPE-FISH (Double Labeling of Oligonucleotide Probes FISH)
DOPE-FISH represents a probe design strategy aimed at enhancing the sensitivity of standard FISH without the complexity of enzymatic amplification. As the name suggests, oligonucleotide probes are labeled with an identical fluorophore at both the 5´- and 3´-end, effectively doubling the number of fluorescent molecules attached to each probe [26]. This straightforward modification yields a nearly twofold increase in sensitivity compared to classic monolabeled FISH probes.
This enhanced sensitivity is advantageous for detecting microorganisms with low ribosomal RNA content or other rare targets. DOPE-FISH has been demonstrated as an effective approach for the simultaneous multicolor detection of six distinct microbial populations in a single assay, facilitating the study of complex microbial community structures [26]. The technique offers a practical balance between improved signal strength and procedural simplicity, serving as a viable alternative when the full amplification power of CARD-FISH is not required.
CLASI-FISH (Combinatorial Labeling and Spectral Imaging FISH)
CLASI-FISH was developed to overcome the multiplexing limitations of conventional FISH, which is typically restricted by the number of spectrally distinct fluorophores. This technique employs a combinatorial labeling strategy, where each microbial taxon is identified not by a single fluorophore, but by a unique combination of fluorophores [19]. Spectral imaging then detects the resulting mixed fluorescence signals, and computational analysis decodes the specific fluorophore combination for each cell, enabling its taxonomic identification.
This approach allows the number of distinguishable taxa to far exceed the number of available fluorophores. Theoretically, with F fluorophores, CLASI-FISH can distinguish up to 2F - 1 different microbial taxa [19]. This extraordinary multiplexing capacity makes CLASI-FISH exceptionally powerful for profiling complex, multi-species microbial communities, such as those found in biofilms, plant rhizospheres, and animal guts, where it can reveal intricate spatial organization and interspecies interactions at micron-scale resolution.
Detailed Experimental Protocols
Protocol for mRNA CARD-FISH in Cyanobacteria
This protocol, adapted for investigating gene expression heterogeneity in cyanobacteria, outlines the steps for detecting specific mRNA transcripts (e.g., rbcL mRNA) at the single-cell level [24].
Workflow for CARD-FISH Protocol
Key Steps:
- Fixation: Preserve cellular morphology and nucleic acids immediately after collection. Typically done using paraformaldehyde (e.g., 1-4% final concentration) for 1-24 hours at 4°C [24] [25].
- Agarose Coating: Immobilize cells on a solid support (e.g., a microscope slide) by embedding them in a thin, low-melting-point agarose film (0.1-0.8%). This step is crucial for maintaining sample integrity during subsequent treatments [24].
- Permeabilization: Render the cell wall permeable to allow probe entry. For cyanobacteria, this involves an enzymatic treatment, often with lysozyme (0.1-10 mg/mL), to degrade the peptidoglycan layer. Incubation conditions (temperature, duration) must be optimized for the specific cyanobacterial species [24].
- Hybridization with HRP-labeled Probe: Apply the specific oligonucleotide probe conjugated to horseradish peroxidase. Hybridization is performed in a buffered saline solution (e.g., containing NaCl, Tris-HCl, formamide, SDS) at an optimized temperature (e.g., 35-46°C) for several hours [24] [26].
- Signal Amplification (Catalyzed Deposition): Incubate the sample with fluorescently labeled tyramide substrates in the presence of hydrogen peroxide. The HRP enzyme catalyzes the covalent deposition of multiple tyramide molecules, leading to significant signal amplification at the probe binding site [26].
- Microscopy and Analysis: Visualize and quantify the fluorescence signals using epifluorescence or confocal microscopy. Image analysis software is then used to assess signal intensity and heterogeneity at the single-cell level [24].
Protocol for Highly Multiplexed SEER-FISH
Sequential error-robust FISH (SEER-FISH) is a cutting-edge method that significantly increases multiplexing capacity through sequential rounds of hybridization and imaging [19]. While related to CLASI-FISH in its goal of high-throughput mapping, it employs a different operational principle.
Table 2: Key Reagents for SEER-FISH and CLASI-FISH Multiplexing
| Reagent / Component | Function / Description | Application Context |
|---|---|---|
| Taxon-Specific Oligonucleotide Probes | Target 16S or 23S rRNA; designed with stringent criteria for specificity [19] | SEER-FISH / CLASI-FISH |
| Fluorophore-Conjugated Reporters | Binds to primary probes; multiple colors available (e.g., FAM, Cy3, Cy5) [19] | SEER-FISH / CLASI-FISH |
| Dissociation Buffer | Removes hybridized probes after imaging, enabling sequential rounds [19] | SEER-FISH |
| Error-Robust Barcode Codebook | Predefined set of barcodes with minimal Hamming distance for error correction [19] | SEER-FISH |
| Combinatorial Probe Labeling Mix | A unique mixture of fluorophores assigned to each microbial taxon [19] | CLASI-FISH |
Workflow for Sequential FISH Protocol
Key Steps:
- Probe Design and Barcode Assignment: Design oligonucleotide probes targeting the 16S or 23S rRNA of the microbial taxa of interest. Each taxon is assigned a unique multi-round barcode from an error-robust codebook [19].
- Sample Preparation: Affix the complex microbial community (e.g., from a plant rhizosphere) onto a glass coverslip and permeabilize cells for probe access [19].
- Sequential Hybridization and Imaging Cycles:
- Hybridization: Apply a pool of probes for the current imaging round.
- Imaging: Capture multichannel fluorescence images.
- Probe Dissociation: Treat the sample with a dissociation buffer to remove the hybridized probes without damaging the sample. This step is repeated for N rounds (e.g., >25 rounds have been demonstrated) [19].
- Image Alignment and Barcode Identification: Align images from all sequential rounds to account for minor shifts. For each bacterial cell, compile its fluorescence profile across all rounds to determine its unique barcode [19].
- Error-Correction and Taxonomic Identification: Use the error-robust encoding scheme (e.g., a minimal Hamming distance of 4) to correct for detection errors and accurately assign taxonomic identity to each cell [19].
- Spatial Profiling: Map the identified taxa back to their original locations to reconstruct the spatial organization of the microbial community at micron-scale resolution and analyze microbial biogeography [19].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Reagents for Advanced FISH Methodologies
| Reagent / Material | Function | Example Variants |
|---|---|---|
| Oligonucleotide Probes | Hybridize to target nucleic acid sequences; backbone of all FISH techniques. | monoProbes, dopeProbes (DOPE-FISH), HRP-Probes (CARD-FISH), Click-labeled probes [26] |
| Fluorophores | Generate detectable fluorescence signal. | FAM, Cyanine 3 (Cy3), Cyanine 5 (Cy5) [26] [19] |
| Horseradish Peroxidase (HRP) | Enzyme for signal amplification in CARD-FISH; catalyzes tyramide deposition [26] | CARD-FISH |
| Tyramide Substrates | Fluorescently-labeled tyramide; substrate for HRP in amplification reaction [26] | CARD-FISH |
| Permeabilization Agents | Disrupt cell walls/membranes to allow probe entry. | Lysozyme (for bacteria) [24], detergent solutions |
| Signal Amplification Systems | Enhance weak fluorescence signals for low-abundance targets. | Tyramide Signal Amplification (TSA) in CARD-FISH [26] |
| N-Isohexadecylacrylamide | N-Isohexadecylacrylamide|Hydrophobic Acrylamide Monomer | N-Isohexadecylacrylamide is a hydrophobic monomer for research on polymers, coatings, and drug delivery. For Research Use Only. Not for human use. |
| 2-Ethylhexyl crotonate | 2-Ethylhexyl crotonate, CAS:7299-92-5, MF:C12H22O2, MW:198.30 g/mol | Chemical Reagent |
The diversification of FISH technologies into specialized variants like CARD-FISH, DOPE-FISH, and CLASI-FISH has profoundly enhanced the toolbox available to researchers studying microbial communities. Each variant addresses a specific set of challenges: CARD-FISH provides the sensitivity required for detecting low-abundance targets and analyzing gene expression, DOPE-FISH offers a simple yet effective sensitivity boost for standard applications, and CLASI-FISH and related sequential methods break through multiplexing barriers to map complex communities.
These advancements allow scientists to move beyond mere detection to conduct sophisticated analyses of microbial community structure, function, and spatial dynamics in their native contexts. The ongoing development of more robust protocols, automated platforms [27], and increasingly multiplexed imaging approaches [19] promises to further solidify the role of FISH technologies as indispensable instruments in microbial ecology, diagnostic pathology, and drug development. The future of microbial detection research will undoubtedly be shaped by the continued integration and refinement of these powerful FISH variants.
Executing FISH: Optimized Protocols and Cutting-Edge Applications in Microbial Detection
Fluorescence in situ hybridization (FISH) is a powerful cytogenetic technique that enables the detection and localization of specific DNA or RNA sequences within intact cells, preserved tissue sections, or chromosomes. By combining molecular genetics with conventional cytogenetics, FISH provides high-resolution spatial and temporal information about genetic abnormalities, gene expression, and microbial identity in complex samples. This application note details the critical steps in the FISH workflow, from sample preparation and fixation through probe hybridization and final visualization, with particular emphasis on its application in microbial detection research. The protocols and methodologies outlined herein are designed to assist researchers, scientists, and drug development professionals in implementing robust and reproducible FISH assays for their investigative and diagnostic needs.
In microbial ecology and diagnostics, FISH is indispensable for identifying, quantifying, and localizing yet-uncultured bacteria within their natural environments. The technique typically targets the highly conserved 16S ribosomal RNA (rRNA), which is present in all living organisms and provides a genetic signature for taxonomic classification. Signature sequences unique to specific taxonomic groups are identified, and complementary DNA probes labeled with fluorescent dyes are synthesized. Under controlled hybridization conditions, these probes bind specifically to the rRNA of target microorganisms, allowing not only for their staining but also for spatial localization within samples such as biofilms, consortia, or attached to surfaces. A critical prerequisite for successful microbial detection is the immediate preservation of samples with a fixative (e.g., formaldehyde) after collection to maintain the natural biocoenosis and spatial distribution of the microbes [28].
Critical Steps in the FISH Workflow
The FISH procedure can be broken down into several key phases, each containing critical steps that influence the success and accuracy of the assay.
Sample Preparation and Fixation
Proper sample preparation is foundational for preserving morphology and nucleic acid integrity.
- Sample Collection: Samples can vary widely, including cultured cells, peripheral blood, bone marrow, urine, Formalin-Fixed Paraffin-Embedded (FFPE) tissues, or environmental samples like water, soil, or biofilms [29] [30]. For microbial samples, immediate fixation is crucial post-collection.
- Fixation: Fixation arrests cellular processes and preserves the sample's structural integrity. Common fixatives include Carnoy's solution for cell pellets or 4% paraformaldehyde for environmental microbes and tissues [29] [28] [31].
- Slide Preparation: Fixed cell pellets are resuspended in fixative and dropped onto microscope slides. For FFPE tissues, sections are mounted on slides, baked, and deparaffinized with organic solvents before the assay [29].
- Pretreatment: Slides may undergo pretreatment steps to enable probe access. This can include digestion with pepsin in HCl or proteinase K to remove proteins, and/or treatment with RNase-free DNase if detecting RNA. This is followed by dehydration through an ethanol series (e.g., 70%, 80%, 95%) [31].
Probe Preparation and Labeling
FISH probes are single-stranded DNA sequences complementary to the target region of interest.
- Probe Design: Probes are designed to be complementary to specific genetic loci, such as genes, centromeres, or, for microbes, signature sequences on the 16S rRNA [29] [28].
- Probe Labeling: Probes must be labeled for detection. Labeling can be direct or indirect.
- Direct Labeling: Fluorophores (e.g., Alexa Fluor dyes) are directly incorporated into the probe via methods like nick translation, PCR, or in vitro transcription. This allows for immediate visualization post-hybridization [32].
- Indirect Labeling: Haptens (e.g., biotin, digoxigenin) are incorporated into the probe. Post-hybridization, the hapten is detected using an enzymatic or immunological detection system (e.g., streptavidin conjugated to horseradish peroxidase - HRP), which then catalyzes the deposition of fluorescent tyramide dyes for signal amplification, as in CARD-FISH (Catalyzed Reporter Deposition FISH) [32] [28].
Table 1: Common FISH Probe Labeling and Detection Methods
| Method | Description | Key Advantage | Common Applications |
|---|---|---|---|
| Direct Labeling | Fluorophore (e.g., Alexa Fluor) directly conjugated to probe [32]. | Rapid protocol, no additional detection steps. | Routine FISH, multiplexing. |
| Indirect Labeling | Probe labeled with a hapten (e.g., biotin, DIG); detected with enzyme-conjugated antibody/streptavidin [32] [31]. | Amenable to signal amplification. | Standard immunohistochemical detection. |
| CARD-FISH | Uses a peroxidase-labeled probe to catalyze the deposition of multiple fluorescent tyramide molecules [28]. | Signal amplification (10-200x), detects low-abundance targets. | Microbes with low rRNA content. |
| ECHO-FISH | Uses probes with a single thiazole orange (TO) homodimer; fluorescence "turns on" upon hybridization [33]. | No washing steps; very fast protocol (25 min). | Gene expression analysis, live-cell imaging. |
Denaturation and Hybridization
This is the core step where the probe binds to its complementary target sequence.
- Denaturation: The double-stranded DNA in the sample and the labeled probe DNA must be separated into single strands. This is typically achieved by co-denaturing the slide and probe together at a high temperature (e.g., 75°C for 2-5 minutes) on a hotplate or automated hybridization unit [29] [31].
- Hybridization: The temperature is lowered to a specific range (often 37°C to 45°C) to allow the single-stranded probe to anneal to its complementary target sequence. This process occurs in a humidified chamber to prevent evaporation and can last from several hours to overnight. The hybridization buffer often contains formamide, which lowers the melting temperature of DNA, allowing for more stringent hybridization conditions that reduce non-specific binding [29] [34] [31].
Post-Hybridization Washing and Detection
Unbound and non-specifically bound probes must be removed to minimize background fluorescence.
- Stringency Washes: Slides are washed in specific buffers to remove excess probe. A common regimen involves a wash in 0.4x SSC (Saline Sodium Citrate) at an elevated temperature (e.g., 72°C), followed by a wash in 2x SSC with a detergent like Tween-20 at room temperature [29] [31]. The temperature and salt concentration of these washes can be adjusted to control the stringency, ensuring only perfectly matched probe-target duplexes remain.
- Signal Detection (for indirect methods): If an indirect labeling method was used, this step involves applying the detection reagent. For example, a streptavidin conjugate linked to HRP or a fluorophore like Cy3 is applied. In CARD-FISH, this is followed by incubation with fluorescently labeled tyramide, which is deposited by HRP, resulting in significant signal amplification [32] [28] [31].
- Counterstaining and Mounting: A fluorescent counterstain, such as DAPI (4',6-diamidino-2-phenylindole), is applied to visualize the cell nuclei or chromosomes. An anti-fade mounting medium is then used to preserve fluorescence before sealing the coverslip [29] [31].
Visualization and Analysis
The final step involves examining the sample and interpreting the results.
- Microscopy: Slides are analyzed using a fluorescence microscope equipped with specific filter sets for the fluorophores used (e.g., DAPI, FITC, Texas Red, Cy3, Cy5) [29].
- Analysis: The fluorescent signals are counted and their patterns interpreted. For instance, in a normal diploid cell, a probe for a single-copy gene would be expected to produce two signals. Abnormalities, such as deletions (one signal), duplications (three or more signals), or translocations (split signals), are identified based on deviation from the expected pattern [29]. For microbial quantification, the number of fluorescent cells is counted relative to the total number of cells stained with DAPI [28] [30].
FISH Experimental Workflow
Advanced FISH Protocols for Microbial Detection
CARD-FISH for Low-Biomass Microbes
For microorganisms with low ribosomal RNA content, standard FISH may yield weak signals. CARD-FISH overcomes this limitation through signal amplification [28].
Detailed Protocol:
- Fixation: Preserve the environmental sample (e.g., water filtrate, biofilm) with paraformaldehyde (final concentration 1-3%) immediately after collection.
- Probe Hybridization: Hybridize with a probe that is labeled with a hapten (e.g., digoxigenin).
- HRP Conjugation: Incubate the sample with an anti-hapten antibody conjugated to Horseradish Peroxidase (HRP).
- Tyramide Signal Amplification: Incubate the sample with fluorescently labeled tyramide. In the presence of hydrogen peroxide (Hâ‚‚Oâ‚‚), the HRP enzyme catalyzes the activation and covalent deposition of multiple tyramide molecules onto nearby proteins, dramatically amplifying the fluorescence signal.
- Visualization: Wash, counterstain with DAPI, and visualize under a fluorescence microscope.
CARD-FISH Signal Amplification
GeneFISH for Linking Function and Taxonomy
GeneFISH combines the detection of specific genes (indicative of function) with ribosomal RNA (for taxonomic identification) at the single-cell level [28].
Detailed Protocol:
- Sample Preparation: Fix cells as for standard FISH.
- Dual Probe Design:
- Design a 16S rRNA-targeted probe labeled with one fluorophore (e.g., Cy3) to identify the cell.
- Design a polynucleotide probe (e.g., dsDNA) targeting the gene of interest (e.g., a key metabolic gene), labeled with multiple haptens (e.g., digoxigenins).
- Hybridization and Detection: Co-hybridize both probes. Detect the gene-specific probe using an anti-digoxigenin antibody conjugated to HRP, followed by tyramide signal amplification with a fluorophore distinct from the 16S rRNA probe (e.g., Alexa Fluor 488).
- Analysis: Visualize with a fluorescence microscope. A cell showing both signals (e.g., Cy3 for taxonomy and Alexa Fluor 488 for the gene) directly links a metabolic function to a specific microorganism.
Essential Reagents and Materials
Successful FISH relies on a suite of specialized reagents and equipment.
Table 2: The Scientist's Toolkit: Essential Reagents for FISH
| Category/Reagent | Function/Purpose | Examples & Notes |
|---|---|---|
| Fixatives | Preserves cellular morphology and immobilizes nucleic acids. | Paraformaldehyde [31], Carnoy's Solution [29], Ethanol [31]. |
| Permeabilization Agents | Creates holes in the cell membrane/wall to allow probe entry. | Pepsin [31], HCl [31]. |
| Labeled Probes | The core reagent that binds specifically to the target sequence. | Directly labeled with Alexa Fluor dyes [32]; indirectly labeled with biotin or digoxigenin [31]. |
| Hybridization Buffer | The solution in which hybridization occurs; controls stringency. | Typically contains formamide, dextran sulfate, SSC, and blocking DNA [31]. |
| Stringency Wash Buffers | Removes unbound and weakly bound probes to reduce background. | SSC solutions at varying concentrations (e.g., 0.4x, 2x) and temperatures [29] [31]. |
| Detection Reagents | For visualizing indirectly labeled probes. | Streptavidin-Cy3 [31], HRP-conjugated antibodies, Tyramide reagents (for CARD-FISH) [32] [28]. |
| Counterstains | Stains all nuclei/chromosomes for overall visualization. | DAPI [29] [31]. |
| Mounting Medium | Preserves the sample and fluorescence under the coverslip. | Anti-fade mounting media [31]. |
Troubleshooting and Quantitative Considerations
Even with a meticulous protocol, challenges can arise. The table below outlines common issues and their solutions. Furthermore, for applications like monitoring microbial population dynamics in bioreactors, moving from relative to absolute quantification is critical. This can be achieved by incorporating a weight-based measurement during sampling (e.g., using dry weight of biofilm or sediment) and applying specific calculation formulas to determine the absolute abundance of target microbes per unit mass or volume [30].
Table 3: Common FISH Challenges and Solutions
| Problem | Potential Cause | Suggested Remedy |
|---|---|---|
| High Background Fluorescence | Incomplete washing, non-specific probe binding, over-digestion during pretreatment. | Optimize wash stringency (temperature/salt concentration); increase formamide concentration in hybridization buffer; titrate permeabilization time [29] [31]. |
| Weak or No Signal | Low probe concentration or labeling efficiency, insufficient target, poor permeabilization, fluorophore quenching. | Use positive control probe; check probe labeling; increase permeabilization time; use signal amplification (CARD-FISH) [28]; protect slides from light [29]. |
| Autofluorescence | Intrinsic fluorescence of sample components (e.g., in environmental samples). | Use different fluorophores whose excitation/emission spectra do not overlap with autofluorescence; perform a control without a probe to assess level of autofluorescence. |
| Inconsistent Signal Between Experiments | Inconsistent denaturation temperature, hybridization time, or wash conditions. | Use calibrated equipment (e.g., ThermoBrite); standardize all incubation times and temperatures; use fresh reagents [29]. |
In Fluorescence In Situ Hybridization (FISH) for microbial detection research, sample preparation represents the most critical determinant of experimental success. Properly prepared specimens preserve microbial morphology, nucleic acid integrity, and tissue architecture, thereby ensuring accurate hybridization and reliable results. This document provides detailed application notes and protocols for handling cells, tissues, and Formalin-Fixed Paraffin-Embedded (FFPE) sections, specifically framed within microbial detection research. Mastery of these techniques provides the foundation for successful visualization of microbial communities within their structural context.
Essential Research Reagent Solutions
The following table details key reagents and materials essential for FISH sample preparation, particularly in the context of microbial detection studies.
Table 1: Essential Research Reagents and Materials for FISH Sample Preparation
| Item | Function in FISH Protocol |
|---|---|
| Tissue Pretreatment Solution | Used during heat pretreatment to expose target nucleic acids by breaking cross-links formed during fixation [35] [36]. |
| Enzyme Reagent | Digests proteins and removes cellular components that may obscure the target DNA/RNA, enhancing probe accessibility [35] [36]. |
| FISH Probes | Fluorescently labeled nucleic acid sequences designed to bind complementary DNA/RNA sequences of target microorganisms [35] [36]. |
| DAPI Antifade | Counterstain that labels all nuclei (host and microbial) and contains antifading agents to preserve fluorescence during microscopy [35] [36]. |
| Phosphate-Buffered Saline (PBS) | An isotonic solution used for washing steps to maintain pH and osmotic balance without damaging cells or tissues [35] [36]. |
| Ethanol Series (70%, 85%, 95%, 100%) | Used for gradual dehydration of samples to preserve morphology and prepare them for hybridization [35] [36]. |
| 0.4x SSC Wash Buffer | A low-salt stringency wash buffer used post-hybridization to remove non-specifically bound probes, reducing background noise [35] [36]. |
| 2x SSC with 0.05% Tween-20 | A higher-salt wash buffer with a detergent used to remove the stringent wash buffer and prepare the slide for mounting [35] [36]. |
FFPE-FISH Protocol for Microbial Detection in Tissue Sections
This protocol is optimized for detecting microorganisms within tissue architectures, crucial for understanding host-microbe interactions.
Slide Preparation
- For FISH, 4μm - 6μm thick FFPE tissue sections should be used [35] [36].
- Slides must be treated with an adhesive before mounting the tissue section to prevent detachment during subsequent procedures [35] [36].
- Throughout the entire procedure, unless otherwise indicated, it is imperative that the tissue section does not dehydrate prematurely [35] [36].
Heat Pretreatment
- Heat 50ml of Tissue Pretreatment Solution (Reagent 1) in a porcelain wash jar or Coplin jar immersed in a water bath until it reaches 98 - 100°C (boiling) [35] [36].
- Boil slides for 30 minutes. Note: Different incubation times may be required depending on tissue fixation and the nature of the microbial target. A 30-minute incubation is a recommended starting point [35] [36].
- Wash in PBS or dHâ‚‚O at room temperature (RT) for 2 x 3 minutes [35] [36].
Enzyme Digestion
- Cover the tissue with 100-200μl of Enzyme Reagent (Reagent 2) for 10 minutes at RT. Note: Depending on the tissue fixative used and the rigidity of the target microbial cell wall, different incubation times may be required. Excessive digestion will cause loss of nuclei and microbial morphology [35] [36].
- Wash in PBS or dHâ‚‚O at RT for 3 x 2 minutes [35] [36].
- Dehydrate slides sequentially in 70%, 85%, 95%, and 100% ethanol for 2 minutes each at room temperature, air dry, and proceed to denaturation and hybridization [35] [36].
Hybridization and Post-Hybridization Washes
- Pre-denaturation: Remove the probe from the freezer, allow it to warm to RT, and mix uniformly. Transfer 10μl - 15μl (depending on tissue size) to a microcentrifuge tube. Prewarm the probe and sample slide on a 37°C (±1°C) hotplate for 5 minutes. Spot the probe onto the sample, apply a coverslip, and seal with rubber solution glue [35] [36].
- Denaturation: Denature the sample and probe simultaneously on a hotplate at 75°C (±1°C) for 5 minutes [35] [36].
- Hybridization: Place the slide in a humid, lightproof container at 37°C (±1°C) overnight [35] [36].
- Post-Hybridization Washes: Carefully remove the coverslip and glue. Immerse the slide in 0.4xSSC (pH 7.0) at 72°C (±1°C) for 2 minutes without agitation. Drain and then immerse in 2xSSC, 0.05% Tween-20 at RT (pH 7.0) for 30 seconds. Drain the slide and apply 10μl - 15μl of DAPI antifade onto each sample. Cover with a coverslip, remove bubbles, and allow the color to develop in the dark for 10 minutes [35] [36].
- Analysis: View with a fluorescence microscope using appropriate filter sets [35] [36].
Live-FISH for Soil Microbiomes: A Protocol for Cultivation Efforts
Live-FISH combines the taxonomic specificity of FISH with the recovery of viable cells, enabling targeted cultivation of elusive microorganisms.
Application Notes on Viability and Taxon Specificity
Recent research evaluating Live-FISH on soil microbiomes reveals critical considerations for microbial detection research [5]:
- The Live-FISH procedure causes a taxon-specific reduction in viability. A study observed a one-order-of-magnitude overall reduction in viable cells [5].
- Planctomycetota and Bacillota have been identified as phyla whose viability is retained to a larger extent throughout the procedure, making them promising targets for Live-FISH and subsequent cultivation [5].
- In contrast, the viability of other dominant phyla like Acidobacteriota can be reduced by up to five orders of magnitude, limiting the applicability of the technique for these taxa [5].
- The method allows for the specific labelling of viable taxa (e.g., planctomycetes), which are then distinguishable in microscopy and flow cytometry analyses, facilitating their sorting for cultivation efforts [5].
Workflow for Live-FISH and Cell Sorting
The following diagram outlines the logical workflow for a Live-FISH procedure aimed at the targeted cultivation of specific microorganisms from a complex microbiome sample.
Quantitative Data for Experimental Planning
Table 2: Impact of Live-FISH on Viability of Soil Microbiome Taxa [5]
| Taxonomic Group | Effect of Live-FISH Treatment on Viability |
|---|---|
| Overall Microbial Community | One-order-of-magnitude reduction in viable cells |
| Planctomycetota | Viability retained to a larger extent (promising target) |
| Bacillota | Viability retained to a larger extent (promising target) |
| Acidobacteriota | Viability reduced by five orders of magnitude |
Table 3: Critical Parameters in FFPE-FISH Protocol [35] [36]
| Protocol Step | Parameter | Recommended Value | Note |
|---|---|---|---|
| Slide Prep | Section Thickness | 4μm - 6μm | |
| Heat Pretreatment | Temperature | 98-100°C | Boiling |
| Duration | 30 minutes | Starting point; varies with fixation | |
| Enzyme Digestion | Duration | 10 minutes at RT | Varies with fixative; avoid over-digestion |
| Denaturation | Temperature & Duration | 75°C (±1°C) for 5 min | Simultaneous with probe |
| Hybridization | Temperature & Duration | 37°C (±1°C) overnight | In humid, lightproof container |
| Stringent Wash | Temperature & Duration | 72°C (±1°C) for 2 min | In 0.4xSSC buffer |
Probe Design and Selection for Maximum Specificity and Sensitivity
In the field of microbial detection research, the efficacy of fluorescence in situ hybridization (FISH) is fundamentally governed by the precision of its core component: the oligonucleotide probe. Achieving maximum specificity and sensitivity is paramount for accurate pathogen identification, gene expression analysis, and cytogenetic studies within complex biological samples [37] [38]. While FISH technology has become a cornerstone technique, its performance is often challenged by off-target binding, which elevates background noise, and insufficient on-target signal, which compromises the detection of low-abundance targets [39] [38].
This application note provides a detailed framework for the design and selection of high-performance FISH probes, with a specific emphasis on applications in microbial detection. We present quantitative design parameters, structured experimental protocols, and a curated toolkit of reagent solutions to empower researchers and drug development professionals to optimize their FISH assays for superior specificity and sensitivity.
Core Principles of FISH Probe Design
The design process must balance two competing objectives: sensitivity, which requires strong binding to the intended target, and specificity, which necessitates minimal interaction with off-target sequences. Key thermodynamic and sequence-based parameters govern this balance.
Probe specificity is critically assessed by evaluating its binding affinity across the entire genome. Advanced design platforms, such as TrueProbes, move beyond simple heuristic filters to perform genome-wide BLAST analyses and thermodynamic modeling. This allows for the ranking of candidate probes based on the difference between on-target and off-target binding energies, thereby systematically minimizing false-positive signals [39]. Furthermore, the complex three-dimensional structure of ribosomal RNA (rRNA)—a common target in microbial FISH—means that not all nucleotide sequences are equally accessible for hybridization, necessitating empirical validation of every newly designed probe [37].
Computational Design Tools and Quantitative Metrics
Several software tools are available for FISH probe design, each employing distinct algorithms and criteria. The table below summarizes and compares the key features of prominent platforms.
Table 1: Comparison of FISH Probe Design Software
| Software | Primary Strategy | Key Metrics | Off-Target Assessment | Notable Features |
|---|---|---|---|---|
| TrueProbes [39] | Genome-wide binding affinity modeling & global ranking | Binding energy difference (on vs. off-target), expressed off-target count | BLAST-based, genome-wide | Incorporates gene expression data; thermodynamic-kinetic simulation |
| Stellaris [39] | Sequential 5' to 3' tiling with heuristic filters | GC content, melting temperature (Tm) | Five masking levels for repetitive sequences | "First-pass" design; widely used for smFISH |
| MERFISH [39] | Hash-based transcriptome screening | Off-target index, rRNA binding score | Comparison of 15/17-mer hashes against transcriptome | Designed for multiplexed error-robust barcoding |
| Oligostan-HT [39] | Energy-based ranking | Gibbs free energy (ΔG°) | Low-complexity screens | Ranks probes by proximity to a user-defined ΔG° optimum |
| PaintSHOP [39] | Alignment plus machine learning | ML-classified probability of off-target duplexes | Bowtie2 alignment | Uses machine learning to triage probe candidates |
The performance of a probe set can be quantified using several metrics. The signal-to-noise ratio (SNR) is a primary measure, where signal is the intensity from specific binding and noise arises from off-target binding and autofluorescence [39]. Detection efficiency refers to the fraction of target RNA molecules that generate a detectable fluorescent spot, a metric where methods using dozens of probes per RNA, like MERFISH, excel [40]. Finally, the false positive rate can be empirically determined using knockout cells or tissues, where any remaining signal is attributable to off-target binding [39].
Experimental Protocol for Probe Validation
The following protocol provides a step-by-step methodology for empirically validating the specificity and sensitivity of a newly designed FISH probe set in a microbial context.
Research Reagent Solutions
Table 2: Essential Reagents for FISH Probe Validation
| Reagent | Function | Example/Note |
|---|---|---|
| Encoding Probes [40] | Target-specific oligonucleotides with readout sequences | Unlabeled DNA probes, 20-50 nt in target region. |
| Fluorescent Readout Probes [40] | Bind to readout sequences on encoding probes to generate signal | Short, fluorescently labeled oligonucleotides. |
| Hybridization Buffer [41] | Creates optimal conditions for probe-target binding | Typically contains salt (e.g., 0.7 M NaCl), buffer (e.g., 0.1 M Tris), and denaturant (e.g., formamide). |
| Formamide [40] | Chemical denaturant that modulates hybridization stringency | Concentration is optimized for each probe set (e.g., 10-40%). |
| Proteinase K [42] | Digest proteins to enhance nucleic acid accessibility for probes. | Critical for tissue permeabilization. |
| Mounting Medium with DAPI [41] | Counterstains nuclei for cellular localization. | - |
Workflow Diagram
The following diagram illustrates the key stages of the FISH protocol, from sample preparation to imaging and analysis.
Step-by-Step Procedure
Sample Preparation and Fixation
- Prepare thin smears of the microbial culture or infected tissue on glass microscope slides.
- Fix cells for 30 minutes at 25°C using 10% neutral buffered formalin [41].
- Permeabilize samples to allow probe access. For tissues, this may involve digestion with Proteinase K [42]. For microbial cells, an ethanol series (50%, 80%, 95% v/v) can be used for dehydration and permeabilization [41].
Pre-hybridization
- Incubate slides in a hybridization buffer without probes to block sites of non-specific binding, thereby reducing background signal [42].
Hybridization
- Prepare the hybridization buffer containing the FISH probe cocktail. A common buffer consists of 0.7 M NaCl, 0.1 M Tris (pH 8.0), 0.1% SDS, and 10 mM EDTA, with a denaturant like formamide [41]. The optimal formamide concentration and hybridization temperature must be determined empirically for each probe set [40].
- Apply the probe solution to the sample and incubate in a moisture-sealed chamber at the hybridization temperature (e.g., 37°C to 55°C) for a defined period. This can range from 30 minutes for some DNA probes [37] to several hours or even days for encoding probes in multiplexed schemes [40].
Post-Hybridization Washes
- Remove unbound and weakly bound probes through a series of stringent washes. Typically, slides are washed with pre-warmed hybridization buffer (without probe) or a buffer with adjusted salt concentration and/or formamide at a specific temperature [41]. This step is critical for minimizing background and enhancing specificity.
Detection and Imaging
- If using direct labeling, proceed to mounting. For indirect labeling (e.g., using haptens like biotin or digoxigenin), incubate with a fluorescently labeled affinity molecule (e.g., streptavidin or an antibody) [42] [32].
- Counterstain nuclei with DAPI [41].
- Mount slides and examine using a fluorescence microscope equipped with appropriate filter sets. For resource-limited settings, LED light sources attached to standard light microscopes can be used effectively [37].
Image and Data Analysis
- Quantify the fluorescence signal intensity and the number of distinct spots per cell using image analysis software.
- Calculate the signal-to-noise ratio by comparing the mean intensity at target sites to the background intensity in non-target areas.
- Assess specificity by comparing signals in positive control samples to those in negative controls (e.g., knockout strains or samples lacking the target pathogen).
Advanced Enhancement Strategies
For challenging applications involving low-abundance targets or highly multiplexed panels, several advanced strategies can be employed to enhance performance.
- Signal Amplification: Techniques such as Tyramide Signal Amplification (TSA), also known as CARD, can dramatically increase sensitivity. This method uses horseradish peroxidase (HRP) to catalyze the deposition of numerous fluorescent tyramine molecules at the probe binding site, resulting in a vastly amplified signal [42] [32]. This is particularly useful for detecting rare transcripts or single-copy genes.
- Throughput Enhancement: Multiplexing using barcoding schemes allows for the simultaneous detection of dozens to thousands of RNA species in a single sample. Methods like MERFISH assign a unique binary barcode of "on" and "off" binding states to each RNA species, which is read out over multiple rounds of hybridization with fluorescent readout probes [40].
- Specificity Enhancement: Split-FISH is a proximity-based method that divides a single probe into two halves. Fluorescence is only activated when both halves bind in close proximity, drastically reducing background from off-target binding [38].
Troubleshooting Common Issues
Table 3: Troubleshooting Guide for FISH Experiments
| Problem | Possible Cause | Solution |
|---|---|---|
| No / Weak Signal | Poor RNA quality; probe concentration too low; overly stringent conditions. | Verify RNA integrity; increase probe concentration; reduce formamide concentration or hybridization temperature [42]. |
| High Background | Probe concentration too high; insufficiently stringent washes; non-specific probe binding. | Decrease probe concentration; increase wash stringency (lower salt, add formamide, raise temperature); pre-screen probes for off-target binding [40] [42]. |
| High Non-Specific Signal in Wrong Cell Types | Tissue-specific adherence or probe cross-hybridization. | Try a different probe target region; adjust hybridization and washing conditions; utilize cell-type-specific markers [42]. |
Robust and reliable FISH assays for microbial detection are built upon a foundation of meticulous probe design and rigorous experimental validation. By leveraging modern computational tools that prioritize genome-wide specificity, adhering to optimized hybridization protocols, and incorporating advanced signal enhancement strategies when necessary, researchers can achieve the high levels of sensitivity and specificity required for cutting-edge research and diagnostic applications. The protocols and guidelines outlined here provide a concrete path toward maximizing the performance of FISH in revealing the spatial organization of microbial communities and host-pathogen interactions.
Fluorescence in situ hybridization (FISH) is a powerful cytogenetic technique that enables the mapping of genetic material within cells and tissues, proving indispensable for both clinical diagnostics and fundamental research [43]. The technique's utility, however, hinges on the critical balance between achieving a strong, specific fluorescent signal from the target sequence and minimizing non-specific background noise. This balance is primarily controlled during two pivotal phases: the hybridization itself and the subsequent post-hybridization stringency washes. Hybridization specificity is driven by the complementarity between the probe and target sequences, as well as parameters like temperature and buffer composition [43]. The post-hybridization washes are equally crucial, as they remove weakly bound, non-specific probes that contribute to background fluorescence, thereby enhancing the signal-to-noise ratio [44]. Within the context of microbial detection, where autofluorescence and complex sample matrices are common challenges, mastering these steps is essential for obtaining reliable and interpretable results. This application note details the protocols and principles for optimizing hybridization and stringency conditions to achieve superior FISH performance in microbial research.
Core Principles of Stringency Control
Stringency in FISH refers to the conditions that favor the dissociation of imperfectly matched (non-specific) probe-target hybrids while preserving perfectly matched (specific) hybrids. This is governed by the thermodynamic stability of the nucleic acid duplex, which is influenced by several key factors [44].
The buffers used for post-hybridization washes are typically based on Saline-Sodium Citrate (SSC), which provides positively charged sodium ions. These ions counteract the natural repulsive negative forces between the phosphate backbones of the probe and target DNA, stabilizing the duplex [44]. The concentration of SSC is inversely related to stringency: a higher SSC concentration (e.g., 2xSSC) provides a low-stringency wash, preserving more hybrids, while a lower concentration (e.g., 0.25xSSC or 0.4xSSC) creates a high-stringency environment, promoting the denaturation of less stable duplexes [44].
Temperature has a direct positive relationship with stringency. Increasing the wash temperature increases the kinetic energy, disrupting the hydrogen bonds holding the duplex together. For most enumeration probes, a wash with 0.25xSSC at 72±1°C for 2 minutes is optimal [44]. The pH of the wash solution also influences stringency by determining the availability of the positive sodium ions [44].
Furthermore, the inclusion of detergents like TWEEN 20 is a key practical consideration. It decreases background staining by preventing non-specific adhesion of probes to glass slides and enhances the uniform spreading of wash reagents over the sample [44].
The following workflow illustrates the logical decision-making process for optimizing stringency washes to balance signal and noise:
Experimental Protocols
Standard Post-Hybridization Wash Protocol for Microbial FISH
This protocol is adapted for common microbial FISH applications, including those using DNA or Peptide Nucleic Acid (PNA) probes, and assumes hybridization has already been performed [44] [45].
Materials:
- Wash Buffer 1: 0.4x SSC or 0.25x SSC, pH ~7.0
- Wash Buffer 2: 2x SSC with 0.05% (v/v) TWEEN 20
- Water bath or dry bath, preheated to 72°C ± 1°C
- Coplin jars or staining dishes
- Forceps
- Coverslips
- Mounting medium with antifade agent (e.g., ProLong Gold)
- DAPI counterstain (if not included in mountant)
Method:
- High-Stringency Wash: Pre-heat Wash Buffer 1 (0.4x or 0.25x SSC) to 72°C. Immediately after removing the hybridization coverslip, immerse the slide in the pre-warmed buffer for 2 minutes with gentle agitation. For PNA FISH, lower salt concentrations and specific temperature optimizations may be required [45].
- Low-Stringency Rinse: Transfer the slide to a Coplin jar containing Wash Buffer 2 (2x SSC/0.05% TWEEN 20) at room temperature. Incubate for 30 seconds to 2 minutes. This step removes residual stringent buffer and begins the process of adding detergent to reduce background.
- Drying and Mounting: Briefly air-dry the slide in a dark place, ensuring the specimen area does not dry completely. Apply an appropriate antifade mounting medium containing DAPI to counterstain the DNA.
- Visualization: Place a coverslip over the specimen and visualize using a fluorescence microscope equipped with appropriate filter sets.
Combined FISH and Immunofluorescence for Cell-Type Identification
This protocol is modified for challenging samples like formalin-fixed paraffin-embedded (FFPE) tissues or cultured neurons, where identifying the cell type expressing a specific miRNA is crucial [46]. It combines LNA probes for superior specificity with tyramide signal amplification (TSA) for sensitivity.
Materials:
- LNA probes (e.g., from Exiqon) digoxigenin-labeled
- DEPC-treated water
- Hybridization buffer: 50% formamide, 5x SSC, 0.04% RNA, 1x Denhardt's solution, 500 µg/mL yeast tRNA, 0.1 mg/mL sheared salmon sperm DNA.
- Tyramide Signal Amplification (TSA) kit (e.g., Cy5 TSA kit from PerkinElmer)
- Anti-Digoxigenin-POD, Fab fragments (Roche)
- Primary and fluorescently-labeled secondary antibodies for immunofluorescence (IF)
- 1-methylimidazole buffer and EDC for post-fixation
- 10mM Sodium citrate buffer, pH 6.0, for antigen retrieval
Method:
- Deparaffinization and Antigen Retrieval: For FFPE sections, deparaffinize in xylene and rehydrate through a graded ethanol series. Perform antigen retrieval by heating slides in 10mM sodium citrate buffer (pH 6.0) [46].
- Post-fixation: Post-fix slides in a solution containing 0.16 M EDC in 1-methylimidazole buffer (pH 8.0) for 1-2 hours at room temperature to prevent loss of small RNAs. Rinse in 0.2% glycine in PBS and then in PBS [46].
- Pre-hybridization and Hybridization: Pre-hybridize with hybridization buffer for 1-2 hours at the hybridization temperature (typically 20-30°C below the probe's Tm). Add digoxigenin-labeled LNA probe to fresh hybridization buffer, apply to the section, and hybridize overnight in a humidified chamber [46].
- Stringency Washes: Wash slides post-hybridization in pre-warmed 2x SSC (for 5 min), 1x SSC (for 10 min), and 0.5x SSC (for 10 min), all at the hybridization temperature, to remove unbound probe [46].
- Signal Amplification and Immunofluorescence:
- Block slides with appropriate buffer (e.g., with BSA).
- Incubate with Anti-Digoxigenin-POD Fab fragments.
- Develop the signal using the Cy5-tyramide substrate from the TSA kit.
- WITHOUT protease treatment, proceed directly to immunofluorescence. Incubate with primary antibody against a cell-type marker (e.g., neuronal marker NeuN), followed by a fluorescently-labeled secondary antibody (e.g., Alexa Fluor 488) [46].
- Counterstaining and Mounting: Counterstain nuclei with DAPI and mount with an antifade mounting medium.
Optimization and Troubleshooting
Achieving the perfect balance between signal and noise often requires fine-tuning. The table below summarizes the effects of key variables and recommends corrective actions for common problems.
Table 1: Troubleshooting Guide for Hybridization and Stringency Washes
| Problem | Potential Cause | Corrective Action |
|---|---|---|
| High Background | Low stringency (Too high SSC, too low temperature) [44] | Increase stringency: Decrease SSC concentration (e.g., to 0.25x) and/or increase wash temperature (e.g., to 73°C) [44]. |
| Insufficient detergent in wash buffer | Ensure TWEEN 20 is added to the final wash buffer (e.g., 0.05%) [44]. | |
| Probe concentration too high; unincorporated nucleotides present [43] | Purify probe to remove unincorporated nucleotides; titrate probe concentration [43]. | |
| Contaminated reagents or equipment [44] | Periodically wash solution jars; use filtered pipette tips; treat work area with DNAse/RNAse eliminating agents [44] [43]. | |
| Weak Signal | Excessive stringency (Too low SSC, too high temperature) [44] | Decrease stringency: Increase SSC concentration (e.g., to 0.4x) and/or decrease wash temperature (e.g., to 71°C) [44]. |
| Inefficient probe penetration or degradation [43] | Optimize permeabilization (e.g., pepsin treatment); use PNA probes for better penetration [43] [45]; ensure proper fixation [43]. | |
| Probe quality or design issues [43] [39] | Verify probe quality (yield, dye incorporation, fragment length); consider using advanced design tools for improved specificity [43] [39]. | |
| Patchy Signal | Uneven reagent distribution during washes; sample drying | Ensure adequate agitation during washes; use detergent (TWEEN 20) to enhance spreading; prevent slides from drying completely [44] [43]. |
The Scientist's Toolkit: Essential Research Reagents
The following table details key reagents and their critical functions in ensuring successful FISH experiments focused on microbial detection.
Table 2: Essential Reagents for FISH in Microbial Detection Research
| Reagent / Material | Function / Application |
|---|---|
| SSC Buffer (Saline-Sodium Citrate) | The foundational buffer for hybridization and washes; provides monovalent cations (Na+) that stabilize nucleic acid hybrids and allows control of stringency via concentration adjustments [44]. |
| Formamide | A denaturing agent used in hybridization buffers to lower the effective melting temperature (Tm) of probes, allowing hybridization to occur at lower, morphologically gentle temperatures [43]. |
| TWEEN 20 Detergent | A non-ionic surfactant added to wash buffers to reduce background fluorescence by preventing non-specific adhesion of probes to glass and ensuring even coverage of solutions [44]. |
| Peptide Nucleic Acid (PNA) Probes | Synthetic DNA mimics with a neutral backbone offering higher binding affinity, better cell penetration, and superior mismatch discrimination compared to DNA probes, ideal for bacterial ID [45]. |
| Locked Nucleic Acid (LNA) Probes | Modified RNA nucleotides with a locked conformation that significantly increases hybridization stability and melting temperature, improving specificity and sensitivity, especially for miRNAs [46]. |
| Tyramide Signal Amplification (TSA) Reagents | An enzyme-mediated signal amplification system that dramatically increases detection sensitivity for low-abundance targets by depositing numerous fluorescent tyramide molecules at the probe site [46]. |
| Antifade Mounting Medium (with DAPI) | Preserves fluorescence by reducing photobleaching during microscopy. DAPI is a blue-fluorescent DNA counterstain that labels nuclei, allowing for the visualization of cellular architecture [43] [46]. |
| Potassium L-alaninate | Potassium L-alaninate, CAS:34237-23-5, MF:C3H6KNO2, MW:127.18 g/mol |
| (Lactato-O1,O2)mercury | (Lactato-O1,O2)mercury|Research Chemical |
Mastering the interplay between hybridization conditions and stringency washes is a cornerstone of robust and reliable FISH analysis in microbial research. By understanding the fundamental principles of how temperature, salt concentration, and pH affect duplex stability, researchers can systematically optimize their protocols to suppress background noise while preserving a strong, specific signal. The protocols and guidelines provided here offer a concrete starting point for this optimization. Adherence to best practices in probe selection, sample preparation, and reagent quality control, as detailed in the provided tables and workflows, will empower scientists to push the boundaries of sensitivity and specificity in their FISH-based detection and diagnostic endeavors.
Multiplexing and Spectral Imaging for Multi-Bacterial Identification
Fluorescence in situ hybridization (FISH) has long been a cornerstone technique for microbial identification and localization in complex samples. A significant limitation of conventional FISH, however, is its low multiplexing capacity, typically allowing only two or three distinct targets to be visualized simultaneously due to spectral overlap of fluorophores and filter-based imaging constraints [47]. The emerging integration of spectral imaging with advanced multiplexing strategies is overcoming this barrier, enabling the simultaneous identification of numerous bacterial taxa in their native spatial context.
These technological advances are particularly crucial for understanding complex microbial communities, such as those in the human microbiome, environmental ecosystems, and host-pathogen interactions. This Application Note details the practical implementation of cutting-edge methods that combine nucleic acid mimics (NAMs), sequential hybridization, error-robust encoding, and computational analysis to achieve highly multiplexed, specific, and quantitative bacterial identification.
Key Technological Approaches
Spectral Imaging with Nucleic Acid Mimics (SI-NAM-FISH)
The SI-NAM-FISH methodology enhances traditional FISH by combining the superior hybridization properties of nucleic acid mimics (NAMs), such as locked nucleic acid (LNA) and 2'-O-methyl-RNA (2'OMe), with the discriminative power of spectral imaging [47].
- Probe Design: NAMs are incorporated into oligonucleotide probes, providing higher thermodynamic stability and specificity compared to DNA probes. This allows for the use of shorter probe sequences and finer control over melting temperatures ((T_m)), which is critical for multiplexing.
- Spectral Image Acquisition: Instead of standard filter-based imaging, full emission spectra are captured for each pixel. Linear unmixing algorithms then deconvolute the signals from multiple fluorophores, even those with overlapping emission spectra [47].
- Implementation: In a foundational study, an LNA/2'OMe EUB338 probe (targeting a universal bacterial rRNA region) was conjugated to seven different fluorochromes (ATTO 550, ATTO 633, ATTO 655, Alexa Fluor 488, Alexa Fluor 405, and others) and tested on seven clinically relevant bacterial species, including Pseudomonas aeruginosa and Escherichia coli [47]. The workflow involves hybridizing the probe cocktail, acquiring spectral images, and computationally unmixing the signals to identify and quantify each bacterial target.
Sequential Error-Robust FISH (SEER-FISH)
SEER-FISH dramatically increases multiplexing capacity by using sequential rounds of probe hybridization and dissociation, coupled with error-correcting barcodes [19].
- Principle: Each bacterial taxon is assigned a unique multi-bit barcode. Over (N) rounds of hybridization, the presence (ON) or absence (OFF) of signal in each of (F) color channels is recorded, yielding a theoretical coding capacity of (F^N) [19].
- Experimental Protocol:
- Sample Preparation: Fix cells or tissue sections on a coverslip.
- Multi-round Imaging: For each round:
- Hybridize with a pool of fluorescent probes targeting specific rRNA sequences.
- Image the sample in all fluorescence channels.
- Dissociate the probes using a dedicated buffer (e.g., 2x SSC with 50% formamide) without damaging the sample [19].
- Image Analysis: Align images from all rounds, segment individual bacterial cells, and extract fluorescence intensity traces to reconstruct the barcode for each cell.
- Error-Robust Encoding: To ensure accuracy, barcodes are designed with a minimum Hamming distance (e.g., HD=4), meaning any two valid barcodes differ in at least 4 bits. This allows the computational correction of 1- or 2-bit errors caused by non-specific hybridization or probe drop-out [19]. This strategy has been shown to achieve a median precision of 0.98 and recall of 0.89 in identifying 12 bacterial species [19].
Signal Amplification by Exchange Reaction FISH (SABER-FISH)
SABER-FISH addresses the challenge of low signal intensity from single-copy targets, which is common in bacterial cells, through in vitro signal amplification.
- Workflow:
- Probe Design: Design primary probes complementary to the target rRNA.
- Concatenemer Synthesis: Using a Primer Exchange Reaction (PER), synthesize long, single-stranded DNA concatemers in vitro that contain many repeats of a specific "imager-binding" sequence. These are appended to the primary probes.
- Hybridization and Imaging: Hybridize the concatemer-linked probes to the sample. Then, fluorescently labeled, short "imager" strands are hybridized to the concatemers, resulting in a greatly amplified signal at the target location [48].
- DNA-Exchange Imaging (DEI): For multiplexing, imagers for different targets can be hybridized, imaged, and then stripped off with a mild washing buffer (e.g., 2x SSC with 8 M urea) without removing the underlying concatemers. This allows sequential imaging of dozens of targets with a limited number of fluorophores [48].
The following diagram illustrates the core logical relationships and workflows of these advanced FISH methodologies.
Research Reagent Solutions
Successful implementation of these advanced imaging techniques relies on a specific toolkit of reagents and probes. The table below catalogs essential materials and their functions.
Table 1: Essential Research Reagents for Multiplexed Bacterial FISH
| Reagent / Material | Function / Description | Application Examples |
|---|---|---|
| LNA/2'OMe Probes [47] | Nucleic acid mimics that confer high binding affinity and specificity to DNA/RNA targets, allowing for stringent hybridization conditions and shorter probe design. | SI-NAM-FISH for robust detection of clinical pathogens like Staphylococcus aureus and Klebsiella pneumoniae. |
| Padlock Probes [49] | Linear oligonucleotides that circularize upon hybridization to a cDNA target. Serve as a template for rolling circle amplification (RCA) to boost signal. | DART-FISH for highly multiplexed RNA mapping in human tissues. |
| SABER Concateners [48] | Long, repetitive DNA strands synthesized in vitro via Primer Exchange Reaction (PER) and appended to FISH probes to provide high-density binding sites for fluorescent imager strands. | SABER-FISH for signal-amplified, multiplexed imaging of RNA and DNA in cells and tissues. |
| Error-Robust Barcode Library [19] | A pre-defined set of multi-bit barcodes with a minimum Hamming distance, enabling correction of identification errors from non-specific probe binding. | SEER-FISH for accurate taxonomic identification of up to 12 bacterial species in complex communities. |
| Fluorophores (e.g., ATTO series, Alexa Fluor series) [47] | Bright, photostable dyes with distinct emission spectra. Selected for high quantum yield and molar extinction coefficient to maximize signal-to-noise ratio. | SI-NAM-FISH; seven fluorophores were ranked by performance on bacterial cells. |
| Dissociation Buffer (e.g., with Formamide) [19] | A buffer solution used to gently strip hybridized probes from rRNA targets between imaging rounds in sequential FISH without damaging the sample integrity. | SEER-FISH for >25 rounds of probe hybridization and dissociation. |
Comparative Performance Data
The quantitative performance of different multiplexed FISH methods is critical for selecting the appropriate technique for a given research goal. The following tables summarize key metrics from the literature.
Table 2: Quantitative Performance of Multiplexed FISH Methods for Bacterial Identification
| Method | Multiplexing Capacity | Reported Accuracy | Key Bacterial Targets Demonstrated | Reference |
|---|---|---|---|---|
| SI-NAM-FISH | 7 species with 7 fluorophores | Correctly distinguished species in validation tests with mixed populations | P. aeruginosa, E. coli, S. aureus, K. pneumoniae, E. faecalis, C. freundii, A. calcoaceticus | [47] |
| SEER-FISH | Theoretically (F^N); 12 species with an R8HD4 codebook | Median Precision: 0.98; Median Recall: 0.89 (after error-correction) | 12 rhizosphere bacterial isolates (e.g., Pseudomonas simiae, Variovorax sp.) | [19] |
| Multiplex FISH (Conventional) | 3 species (typical maximum) | Identified coinfections in 3% of sampled fish | Myxobolus inornatus, Flavobacterium spp., Aeromonas spp. in Smallmouth Bass | [50] |
Table 3: Fluorophore Performance Ranking in Bacterial FISH
| Fluorophore | Relative Fluorescence Intensity on E. coli* | Notable Properties |
|---|---|---|
| ATTO 550 | High | High brightness and photostability [47] |
| ATTO 633 | High | High brightness and photostability [47] |
| Alexa Fluor 488 | Low | Lower intensity under tested conditions [47] |
| Alexa Fluor 405 | Low | Lower intensity under tested conditions [47] |
| ATTO 655 | Low | Lower intensity under tested conditions [47] |
- Ranking based on data from [47], where an EUB338 LNA/2'OMe probe conjugated to different fluorophores was hybridized to E. coli.
Detailed Experimental Protocol: SEER-FISH for Spatial Microbial Profiling
This protocol, adapted from [19], outlines the steps for performing SEER-FISH to identify multiple bacterial species on a root surface or similar substrate.
Sample Preparation and Fixation
- Cultivation: Grow microbial communities of interest on the substrate (e.g., plant roots, a coverslip).
- Fixation: Immerse the sample in 4% paraformaldehyde (PFA) in 1x PBS for 1 hour at room temperature.
- Permeabilization: Treat the sample with a permeabilization solution (e.g., 1 mg/mL Lysozyme in 1x PBS) for 10-30 minutes at 37°C to facilitate probe entry.
- Dehydration: Dehydrate the sample through an ethanol series (50%, 80%, 98% for 3 minutes each) and air dry.
Multi-round FISH Imaging
- Codebook Design: Assign a unique (N)-bit barcode from an error-robust codebook (e.g., R8HD4) to each target bacterial species.
- Probe Hybridization: For each round of imaging (i) (where (i) = 1 to (N)):
- Prepare a hybridization buffer containing the relevant species-specific probes (e.g., 50% deionized formamide, 2x SSC, 10% dextran sulfate, 0.1% SDS).
- Apply the buffer to the sample, cover with a HybriWell chamber, and incubate in a humidified chamber at 40°C for 30-45 minutes.
- Washing: Remove the hybridization buffer and wash the sample with pre-warmed wash buffer (e.g., 2x SSC with 50% formamide) for 10-15 minutes at 40°C, followed by a brief rinse in 1x PBS.
- Imaging: Mount the sample in an anti-bleaching buffer and acquire images using a fluorescence microscope with the appropriate filter sets for all (F) color channels. Maintain consistent exposure times across rounds.
- Probe Dissociation: Remove the coverslip and immerse the sample in dissociation buffer (e.g., 2x SSC with 50% formamide) for 15-30 minutes at 40°C to remove the hybridized probes.
- Repetition: Repeat steps 2-5 for all (N) rounds of the experiment.
Image Analysis and Decoding
- Image Registration: Use computational tools to align all images from the (N) rounds to correct for any stage drift.
- Cell Segmentation: Apply a segmentation algorithm (e.g., watershed) to the composite image to define the boundaries of individual bacterial cells.
- Barcode Extraction: For each segmented cell, extract a fluorescence intensity vector across all rounds and channels. Normalize the intensities and assign an ON or OFF bit for each channel in each round.
- Error-Correction and Identification: Compare the extracted (N)-bit barcode to the valid barcodes in the codebook. Identify the cell's taxonomy by finding the valid barcode with the smallest Hamming distance, applying error-correction as needed [19].
The integration of spectral imaging and sophisticated multiplexing strategies is revolutionizing our ability to identify and spatially map complex bacterial communities. Techniques like SI-NAM-FISH, SEER-FISH, and SABER-FISH, each with unique strengths in simultaneous detection, ultimate scalability, and signal amplification, provide a powerful toolkit for researchers. By following the detailed protocols and leveraging the specified reagent solutions, scientists and drug development professionals can now probe microbial ecosystems with unprecedented depth and precision, accelerating research in infectious disease, microbiome science, and microbial ecology.
Application in Oral Microbiome Research and Ecosystem Analysis
Fluorescence in situ hybridization (FISH) is a powerful, culture-independent technique that has revolutionized the analysis of complex microbial ecosystems, including the oral microbiome [11]. By using fluorescently labeled nucleic acid probes that hybridize to specific ribosomal RNA (rRNA) sequences within intact microbial cells, FISH allows for the simultaneous identification, quantification, and spatial localization of microorganisms in their native habitat [8] [11]. For oral microbiome research, this provides critical insights into the composition, organization, and dynamics of dental plaque biofilms, and their role in health and disease, offering significant advantages over methods that require cultivation [11].
Core Principles and Advantages of FISH for Oral Microbiology
The application of FISH in oral microbiology typically targets the 16S or 23S ribosomal RNA (rRNA) genes [11] [45]. These genes are ideal targets because they contain both highly conserved regions, which facilitate broad-based detection, and variable regions, which allow for the design of probes specific to different taxonomic levels (e.g., genus or species) [45]. The high copy number of rRNA molecules within each bacterial cell also naturally amplifies the signal, making detection sensitive and straightforward using fluorescence microscopy [11].
FISH offers several critical advantages for studying the oral ecosystem [11]:
- Culture-Independence: It enables the detection and identification of the estimated 30% of oral bacteria that are unculturable, providing a more complete picture of the microbial community [11].
- In Situ Analysis: It allows for the direct visualization of microbial spatial organization and inter-species interactions within intact oral biofilms, which is lost when samples are homogenized for DNA extraction [11].
- High Specificity and Sensitivity: With proper probe design, FISH can accurately distinguish between closely related bacterial species [11].
- Rapid Detection: The protocol can be completed within a few hours, allowing for relatively quick analysis compared to traditional culture methods [11].
Recent technological variants have further enhanced its capabilities. Peptide Nucleic Acid (PNA) probes, which use an uncharged pseudopeptide backbone, offer higher binding affinity, better penetration through bacterial cell walls, and greater specificity due to higher sensitivity to mismatches compared to DNA probes [45]. For complex multi-species detection, methods like combinatorial labeling and spectral imaging FISH (CLASI-FISH) enable the simultaneous visualization of dozens of different taxa in a single sample [11].
Detailed FISH Protocol for Oral Microbiome Analysis
The following section provides a detailed core protocol for FISH, optimized for the analysis of oral biofilm samples such as dental plaque. This protocol can be adapted for use with DNA or PNA probes.
Sample Collection and Fixation
- Sample Collection: Using a sterile curette or toothpick, collect supragingival or subgingival plaque from the tooth surface. Transfer the sample immediately into a sterile microcentrifuge tube containing 1 mL of 1X phosphate-buffered saline (PBS).
- Dispersion: Vortex the sample briefly to homogenize the plaque biofilm.
- Fixation: Add 3 volumes of 4% paraformaldehyde (PFA) solution to the sample and incubate at 4°C for 4-12 hours. This step cross-links and preserves the cellular morphology and nucleic acids.
- Washing: Pellet the cells by centrifugation at 10,000 × g for 5 minutes. Carefully remove the PFA supernatant and wash the pellet twice with 1 mL of 1X PBS.
- Storage: Resuspend the final pellet in 1 mL of a 1:1 mixture of 1X PBS and absolute ethanol. Fixed samples can be stored in this buffer at -20°C for several months.
Sample Immobilization and Permeabilization
- Spotting: Apply 10-20 µL of the fixed cell suspension onto a clean, charged glass microscope slide and allow it to air dry completely.
- Dehydration: Immerse the slide in a series of ethanol baths (50%, 80%, and 96%) for 3 minutes each, then allow it to air dry.
- Permeabilization (Critical for Gram-positive bacteria): To enhance probe penetration, especially for Gram-positive oral species, treat the sample with lysozyme (10 mg/mL in 0.1 M Tris-HCl, 50 mM EDTA, pH 8.0) for 10-60 minutes at 37°C. Subsequently, wash the slide briefly with distilled water and dehydrate again through the ethanol series.
Hybridization
- Probe Solution: Prepare the hybridization buffer. A standard buffer contains 0.9 M NaCl, 20 mM Tris-HCl (pH 7.2), 0.01% SDS, and formamide (the concentration of which is probe-specific and determines hybridization stringency). Add the fluorescently labeled probe to a final concentration of 5-50 ng/µL.
- Hybridization: Apply 20-30 µL of the probe solution to the dried sample area and immediately cover with a coverslip to prevent evaporation.
- Incubation: Place the slide in a pre-warmed, humidified chamber and incubate in the dark at the appropriate hybridization temperature (typically 45-55°C for DNA probes, or 55-65°C for PNA probes) for 90-120 minutes [45].
Washing and Detection
- Washing: Remove the coverslip and immerse the slide in a pre-warmed washing buffer (e.g., for a standard buffer: 20 mM Tris-HCl (pH 7.2), 5 mM EDTA, 0.01% SDS, and NaCl concentration determined by the formamide concentration in the hybridization buffer). Incubate at 48°C for 15-30 minutes.
- Rinsing: Briefly rinse the slide with cold distilled water and air dry in the dark.
- Counterstaining and Mounting: Apply a mounting medium that contains a general nucleic acid counterstain, such as DAPI (4',6-diamidino-2-phenylindole), to visualize all cells. Apply a coverslip and seal with clear nail polish.
- Microscopy: Examine the slide using an epifluorescence or confocal laser scanning microscope equipped with appropriate filter sets for the fluorophores used.
The Scientist's Toolkit: Key Research Reagent Solutions
The successful implementation of FISH relies on a set of key reagents and materials. The following table details essential components for a FISH experiment targeting oral bacteria.
Table 1: Essential Reagents and Materials for FISH in Oral Microbiome Research
| Item | Function/Description | Example/Note |
|---|---|---|
| Nucleic Acid Probes | Target-specific oligonucleotides labeled with a fluorophore for detection. | DNA oligonucleotides (~20 bases) or PNA probes (15-18 bases) [45]. |
| Paraformaldehyde (PFA) | Fixative that preserves cellular morphology and immobilizes nucleic acids. | Typically used at 4% in PBS. |
| Ethanol Series | Used for dehydration and storage of samples; also aids in permeabilization. | 50%, 80%, and 96% or absolute ethanol. |
| Lysozyme | Enzymatic permeabilization agent critical for digesting the peptidoglycan layer of Gram-positive bacteria. | 10 mg/mL in Tris-EDTA buffer, incubation at 37°C [11]. |
| Formamide | Denaturing agent used in hybridization buffer to control stringency and specificity. | Concentration is probe-specific (0-50%); higher concentration increases stringency. |
| Hybridization Buffer | Provides ionic strength and appropriate chemical environment for specific probe binding. | Contains salts (e.g., NaCl), buffer (e.g., Tris-HCl), detergent (e.g., SDS), and formamide. |
| Mounting Medium with DAPI | Preserves the sample for microscopy and provides a general counterstain to visualize total microbial cells. | DAPI stains double-stranded DNA, fluorescing blue. |
| Tin(2+) acrylate | ||
| 2-Octyldodecyl heptanoate | 2-Octyldodecyl heptanoate, CAS:94277-33-5, MF:C27H54O2, MW:410.7 g/mol | Chemical Reagent |
Quantitative Performance Data and Advanced Applications
Advanced FISH methodologies, particularly those employing PNA probes, demonstrate exceptional performance in identifying bacterial species. The quantitative data below highlights the specificity and accuracy achievable with optimized FISH protocols.
Table 2: Performance Metrics of PNA-FISH for Identification of Common Oral Bacteria
| Bacterial Species | Probe Type | Reported Identification Accuracy | Key Factor for Performance |
|---|---|---|---|
| Escherichia coli | PNA Probe | 99.9% | High mismatch sensitivity and binding affinity of PNA [45]. |
| Pseudomonas aeruginosa | PNA Probe | 99.8% | Superior penetration through bacterial membranes [45]. |
| Staphylococcus aureus | PNA Probe | 99.7% | Ability to hybridize at low salt concentrations, destabilizing rRNA secondary structure [45]. |
| Klebsiella pneumoniae | PNA Probe | 99.5% | Optimal probe design based on full 16S rRNA sequence analysis [45]. |
| Enterococcus faecalis | PNA Probe | 98.5% | FRET-based detection eliminating cross-talk between species [45]. |
| Bacillus subtilis | PNA Probe | 97.5% | Use of cleavable fluorophores for sequential multi-species identification [45]. |
| Proteus mirabilis | PNA Probe | 96.0% | Specificity achieved by targeting regions that maximize mismatches with non-target species [45]. |
Advanced applications combine FISH with other techniques to extract more information. FISH-immunofluorescence (FISH-IF) allows for the simultaneous detection of microbial identity and host or microbial proteins, useful for studying host-pathogen interactions [8]. CLASI-FISH uses combinatorial labeling with multiple fluorophores to dramatically increase the number of taxa that can be identified in a single sample, revealing complex community structures in dental plaque [11]. FRET-based FISH employs two probes binding to adjacent sites on the rRNA, where a signal is only generated if both bind, virtually eliminating false positives from non-specific probe binding [45].
Workflow and Signaling Pathway Diagrams
FISH Experimental Workflow
The following diagram illustrates the key procedural steps in a standard FISH protocol, from sample collection to final microscopy.
PNA Probe Hybridization Mechanism
This diagram details the molecular mechanism of how PNA probes hybridize to their target rRNA sequences and the principle of FRET-based detection for enhanced specificity.
Application Note
Fluorescence in situ hybridization (FISH) is a powerful cytogenetic technique that uses fluorescently labeled DNA probes to detect and localize specific nucleic acid sequences within cells, providing critical information for clinical diagnostics [51] [52]. This application note details its use in the context of bacteremia and the development of pathogen-specific probes, highlighting the technical rigor required for clinical implementation.
The clinical utility of FISH is particularly evident in its ability to provide rapid pathogen identification directly from patient samples, which is crucial for conditions like bacteremia and sepsis where timely intervention is critical [52]. While initially developed for human cytogenetics, the principles of FISH have been successfully adapted for microbial detection, allowing for the simultaneous visualization of morphology and genetic identity of pathogens. The technique can be performed on various sample types, including blood cultures, formalin-fixed paraffin-embedded (FFPE) tissues, and directly on smears, offering versatility in the diagnostic workflow [8] [52]. The use of pathogen-specific probes enables the precise identification of etiological agents, guiding appropriate antimicrobial therapy and improving patient outcomes.
Experimental Protocols
Protocol 1: FISH for Direct Detection from Blood Culture
This protocol is designed for the rapid identification of pathogens directly from positive blood culture bottles, reducing the time to result compared to traditional culture methods.
Sample Preparation:
- Take a 1-2 mL aliquot from a signal-positive blood culture bottle.
- Centrifuge at 12,000 × g for 5 minutes to pellet microbial cells.
- Wash the pellet twice with sterile phosphate-buffered saline (PBS).
- Apply the cell suspension onto a clean glass slide and allow to air dry.
- Fix cells by immersing the slide in 4% paraformaldehyde for 10 minutes, followed by dehydration in an ethanol series (70%, 80%, 96%) for 3 minutes each [51] [52].
Probe Hybridization:
- Prepare the hybridization mix containing the pathogen-specific FISH probe (e.g., at a concentration of 5-10 ng/μL) in a buffer containing formamide and saline-sodium citrate (SSC).
- Apply 10-20 μL of the hybridization mix to the fixed sample on the slide and cover with a coverslip.
- Denature the probe and target DNA simultaneously on a heated block or in a hybridizer at 85°C for 5 minutes.
- Immediately transfer the slide to a humidified chamber and incubate at 46°C for 90-120 minutes to allow for hybridization [51] [8].
Washing and Detection:
- Carefully remove the coverslip and wash the slide in a pre-warmed washing buffer (e.g., 48°C) to remove unbound probe.
- Briefly rinse the slide in cold distilled water and air dry in the dark.
- Mount the slide using an antifading mounting medium containing DAPI (4',6-diamidino-2-phenylindole) to counterstain all nucleic acids [52].
Visualization and Analysis:
- Analyze the slide using a fluorescence microscope equipped with appropriate filter sets for the fluorophores used.
- Score for the presence of brightly fluorescing microbial cells with morphology consistent with the target pathogen (e.g., cocci in clusters for Staphylococcus aureus) against the DAPI background [51] [52].
Protocol 2: Validation of a Novel Pathogen-Specific FISH Probe
This protocol outlines the key steps for validating a "home-brewed" or novel commercial FISH probe before its introduction into clinical use, ensuring analytical reliability.
Determination of Analytical Specificity and Sensitivity:
- Specificity: Hybridize the probe against a panel of reference strains, including the target species and closely related non-target species. Analytical specificity is calculated as the percentage of loci where the probe hybridizes only to its correct target sequence without cross-hybridization [53]. A value of 100% is the target for clinical-grade probes [53].
- Sensitivity: Hybridize the probe to normal specimens negative for the target abnormality. Analytical sensitivity is the percentage of scorable cells or nuclei showing the expected normal signal pattern and should exceed 98% for reliable probes [53].
Establishing the Normal Cut-off Value:
- Test the probe on a minimum of 20-25 karyotypically normal or target-negative samples.
- For each sample, score a predefined number of interphase cells (e.g., 200-500) for the presence of abnormal signal patterns.
- Calculate the cut-off value (upper reference limit) using a statistical method such as the BETAINV function, which represents the threshold above which a sample is considered positive for the abnormality with a defined confidence level (typically 95% or 99%) [54] [53].
Reproducibility Testing:
- Assess the probe's performance across multiple variables: different days, different operators, different testing sites, and different lots of the probe.
- Use both negative and high-positive class cell samples for these assessments.
- The acceptance criterion is generally >95% agreement across all variables, demonstrating the test's robustness [53].
The workflow for probe validation is a multi-stage process, as summarized below:
Data Presentation
The validation of clinical FISH probes requires the collection of extensive performance data. The following tables summarize key parameters based on a multi-site study of FDA-cleared FISH probes [53].
Table 1: Performance Characteristics of Clinical FISH Probes
| Parameter | Definition | Target Performance | Study Findings [53] |
|---|---|---|---|
| Analytical Specificity | Percentage of signals hybridizing to the correct locus only. | >95% | 100% (95% CI lower limit: 98.12%) |
| Analytical Sensitivity | Percentage of negative cells showing the expected normal signal pattern. | >95% | >98% for all probes evaluated |
| Intra-day Reproducibility | Consistency of results within the same day. | >95% agreement | Met or exceeded 95% |
| Inter-day Reproducibility | Consistency of results across different days. | >95% agreement | Met or exceeded 95% |
| Inter-site Reproducibility | Consistency of results across different laboratories. | >95% agreement | Met or exceeded 95% |
| Inter-lot Reproducibility | Consistency of results across different probe batches. | >95% agreement | Met or exceeded 95% |
Table 2: Stability Profile of Validated FISH Probes
| Stability Factor | Test Conditions | Outcome |
|---|---|---|
| Shelf Life | Storage at -20°C in the dark | Stable for 24 months |
| Freeze/Thaw Cycles | 11 rounds of thawing and freezing | Stable after 11 cycles |
| Transport Conditions | 40°C for two weeks | Demonstrated stability |
| Hybridized Slide | Stored in darkness at 2-8°C | Reproducible analysis for up to one month |
The Scientist's Toolkit
Successful implementation of FISH in clinical and research settings depends on a suite of essential reagents and materials.
Table 3: Research Reagent Solutions for FISH
| Item | Function / Description |
|---|---|
| Pathogen-Specific DNA Probes | Analyte-specific reagents (ASRs) designed to hybridize with complementary sequences in the microbial genome; can be labeled directly with fluorophores (e.g., FITC, Cy3) or indirectly with haptens (e.g., biotin, digoxigenin) [54] [51]. |
| DAPI (4',6-diamidino-2-phenylindole) | A fluorescent counterstain that binds strongly to AT-rich regions in DNA, staining all nuclei and providing a morphological context for the specific FISH signals [52]. |
| Hybridization Buffer | A solution containing formamide, saline-sodium citrate (SSC), and dextran sulfate; formamide denatures DNA and lowers the melting temperature, while SSC controls the stringency of hybridization [51]. |
| Antifading Mounting Medium | Used to mount coverslips onto hybridized slides; reduces photobleaching of the fluorophores during microscopy, preserving signal intensity [52]. |
| Stringent Wash Buffer | A buffer with a specific pH and salt concentration (often involving SSC) used post-hybridization to remove probes that are bound non-specifically, thereby reducing background noise [51] [52]. |
| 3-Propylhept-2-enal | 3-Propylhept-2-enal, CAS:84712-89-0, MF:C10H18O, MW:154.25 g/mol |
| Ilamycin A | Ilamycin A, CAS:11006-41-0, MF:C54H75N9O12, MW:1042.2 g/mol |
Bloodstream infections (BSIs) and sepsis represent severe medical conditions, with approximately 50 million global cases annually and an associated 8% decrease in survival rate for every hour of delayed effective treatment in septic shock [55]. Traditional, culture-based diagnostic methods require 36 to 72 hours for a complete turnaround, creating a critical window of diagnostic uncertainty that often leads to empiric, broad-spectrum antibiotic therapy [56]. This practice contributes to antibiotic resistance, patient toxicity, and suboptimal outcomes [55].
Culture-free diagnostic methods are therefore paramount for advancing patient care. Among these, Fluorescence In Situ Hybridization (FISH) is a powerful technique for the rapid, specific identification of microorganisms without the need for cultivation [11]. This Application Note details the integration of FISH into rapid diagnostic workflows, providing structured protocols, performance data, and essential resources for researchers and scientists developing next-generation diagnostic solutions.
Application Notes: The Role of FISH in Modern Diagnostics
FISH is a cytochemical technique that uses fluorescently labeled nucleic acid probes to hybridize with complementary target sequences within intact microbial cells, allowing for their genetic detection, identification, and spatial localization [11]. Its principal advantages include:
- Rapidity and Specificity: FISH facilitates direct identification from clinical samples, bypassing the 16-48 hour culture step required by most standard methods [11] [56].
- Visualization of Microbial Communities: The technique can reveal the spatial organization and distribution of different microbial communities within a biofilm or tissue sample, providing ecological insights [11].
- Adaptability: Numerous FISH variants have been developed to enhance its capabilities to overcome challenges like low signal intensity or the need for multiplexing.
Key FISH Variants and Their Characteristics
The table below summarizes several key FISH variants, their methodological basis, and their primary applications.
Table 1: Key Variants of FISH Technology
| FISH Variant | Methodological Basis | Primary Applications | Key Advantages |
|---|---|---|---|
| CARD-FISH (Catalyzed Reporter Deposition) | Enzyme-mediated signal amplification using horseradish peroxidase [11]. | Detection of microbes with low ribosomal content; environmental samples [11]. | Greatly enhanced signal intensity [11]. |
| CLASI-FISH (Combinatorial Labeling and Spectral Imaging) | Uses binary combinations of fluorophores to label probes, with spectral imaging for deconvolution [11]. | High-throughput multiplexing for complex microbial communities [11]. | Can distinguish dozens of microbial taxa simultaneously in a single sample [11]. |
| DOPE-FISH (Double Labeling of Oligonucleotide Probes) | Two fluorophores attached to a single probe [11]. | General microbial detection and quantification. | Doubles signal intensity, improving detection reliability [11]. |
| PNA-FISH (Peptide Nucleic Acid FISH) | Uses synthetic DNA mimics with an uncharged peptide backbone as probes [11]. | Detection in samples with high background fluorescence; complex clinical matrices. | Higher affinity and specificity; resistance to enzymatic degradation [11]. |
| RING-FISH (Recognition of Individual Genes FISH) | Uses polynucleotide probes targeting single-copy genes [11]. | Detection of specific bacterial strains based on functional genes. | Enables detection beyond ribosomal RNA targets [11]. |
Experimental Protocols
This section provides a foundational protocol for FISH, adaptable for various sample types.
Core FISH Protocol for Microbial Detection
The following protocol is adapted from methods used to detect bacteria within microarthropods and other samples [11] [57].
A. Specimen Fixation and Preparation
- Fixative: Fix samples in a 4% (wt/vol) paraformaldehyde solution in phosphate-buffered saline (PBS). For hydrophobic samples, add 1% (vol/vol) Triton X-100 and degas the solution to prevent air bubble trapping [57].
- Incubation: Fix for a minimum of 4 hours, or preferably overnight, at 4°C [57].
- Washing: After fixation, wash the specimen twice with degassed PBS to remove residual fixative.
B. Embedding and Sectioning
- Embedding: For delicate specimens, embed in a gelatin-glycerin solution (16g gelatin, 18.9g glycerin, 70ml distilled water). Harden the embedding medium by incubating with a 2% chromium(III) potassium sulfate solution on ice for 1 hour [57].
- Sectioning: Prepare cryosections (0.5 - 5 µm thick) using a cryostat. Transfer sections onto electrostatically charged or adhesive-coated microscope slides [57].
- Dehydration: Dehydrate the sections by passing them through an increasing ethanol series (e.g., 50%, 80%, 96%, 100%) [57].
C. Hybridization
- Hybridization Buffer: Prepare buffer containing 20 mM Tris-HCl (pH 8.0), 0.9 M NaCl, 0.01% sodium dodecyl sulfate, and a concentration of formamide appropriate for the probe's stringency (e.g., 30%) [57].
- Probe Application: Apply 50-150 µL of hybridization buffer containing the labeled probe (e.g., 50 pmol/mL of a Cy3-labeled EUB338 probe for most Bacteria) onto the section [57].
- Incubation: Place a coverslip over the sample and incubate in a dark, humidified chamber at the appropriate hybridization temperature (e.g., 46°C for many 16S rRNA probes, or room temperature) for 1.5 to 3 hours, or overnight [11] [57].
D. Washing and Visualization
- Washing: Remove the coverslip and wash the slide in a pre-warmed wash buffer (e.g., 1x SSC) for 10-15 minutes to remove unbound probe [57].
- Drying and Mounting: Air-dry the slide and mount with an anti-fading agent (e.g., Entellan) [57].
- Microscopy: Observe hybridization signals using an epifluorescence microscope equipped with appropriate filter sets for the fluorophore used (e.g., Cy3 filter set: excitation 546 nm / emission 570 nm) [57].
Integrated Workflow for Culture-Free Sepsis Diagnosis
The diagram below illustrates a rapid, culture-free diagnostic workflow that integrates a bacterial separation step with FISH-based identification, addressing the bottleneck of traditional blood culture.
Diagram 1: Culture-free sepsis diagnosis workflow.
This workflow, combining separation and FISH, can be completed in a few hours, significantly faster than the 36-72 hours required for culture-based methods [55] [56].
Performance Data
Quantitative Comparison of Culture-Free Methods
The table below summarizes the performance of emerging culture-free technologies, including FISH-based approaches, compared to a novel electrochemical immunoassay.
Table 2: Performance Metrics of Selected Culture-Free Diagnostic Platforms
| Platform / Method | Detection Principle | Time to Result | Limit of Detection (CFU/mL) | Key Performance Highlights |
|---|---|---|---|---|
| FISH with Microfluidic Trapping [55] | Bacterial separation via smart centrifugation & microfluidics; detection via deep learning on microscopy images. | ~2 hours | E. coli: 9 CFU/mLK. pneumoniae: 7 CFU/mLE. faecalis: 32 CFU/mL | High separation efficiency; enables direct phenotypic AST after detection. Recovery of S. aureus is low (8%) [55]. |
| RDAP (Rapid Detection-Analysis Platform) [56] | Electrochemical sandwich immunoassay with voltage-controlled signal amplification (FEED). | Detection/ID: 88 minAST: 148 min per antibiotic | 4 CFU/mL | Clinical sample validation: 93.3% accuracy for detection/ID; 95.4% for AST. At least 15h faster than standard care [56]. |
| Integrated Droplet Digital Detection [56] | DNAzyme sensor with droplet microfluidics and a 3D particle counter. | 90 min to 4 h | 1 CFU/mL | Requires diluted blood samples, which may introduce inaccuracy and extra preparation time [56]. |
The Scientist's Toolkit
Successful implementation of culture-free FISH diagnostics requires specific reagents and materials. The following table details essential components.
Table 3: Research Reagent Solutions for FISH-Based Detection
| Item | Function / Application | Example / Note |
|---|---|---|
| Nucleic Acid Probes | Hybridize to target sequences (e.g., 16S rRNA) for specific detection. | EUB338: Targets most Bacteria [57]. Can be labeled with Cy3, FITC, or other fluorophores. |
| Fixative | Preserves cellular morphology and immobilizes nucleic acids. | 4% Paraformaldehyde in PBS is standard. Ensure adequate fixation time [57]. |
| Hybridization Buffer | Creates optimal conditions for specific probe binding. | Contains salts (e.g., NaCl), buffer (e.g., Tris-HCl), and denaturants (e.g., formamide) to control stringency [57]. |
| Formamide | Denaturing agent used in hybridization buffer to control stringency. | Higher concentrations lower the hybridization temperature, increasing probe specificity. |
| Blocking Reagents | Reduce non-specific binding of probes. | Can include sheared salmon sperm DNA or specific blocking oligonucleotides. |
| Mounting Medium | Preserves fluorescence and prepares sample for microscopy. | Use anti-fading mounting media (e.g., Entellan, Vectashield) to slow photobleaching [57]. |
| Fluorophore-Labeled Antibodies | For signal amplification in CARD-FISH. | Horseradish peroxidase (HRP)-labeled antibodies used with fluorescent tyramide substrates [11]. |
| 9-Oxotridecanoic acid | 9-Oxotridecanoic acid, CAS:92155-74-3, MF:C13H24O3, MW:228.33 g/mol | Chemical Reagent |
| Didecylbenzene | Didecylbenzene, CAS:33377-22-9, MF:C26H46, MW:358.6 g/mol | Chemical Reagent |
Beyond the Protocol: Troubleshooting Common FISH Challenges and Enhancing Performance
Diagnosing and Resolving Poor or Absent Fluorescent Signal
In the context of microbial detection research, a poor or absent fluorescent signal in Fluorescence In Situ Hybridization (FISH) can compromise data quality and lead to false negatives. Effective troubleshooting is fundamental to obtaining reliable, reproducible results. This application note provides a structured framework for diagnosing and resolving the most common causes of signal failure in FISH experiments, equipping researchers with practical protocols and optimization strategies.
Core Problem Categories and Solutions
The primary causes of weak FISH signals can be categorized into probe penetration issues, target accessibility problems, and detection sensitivity limitations. The following table summarizes these core problems and their respective solutions.
Table 1: Common Causes of Poor FISH Signal and Recommended Solutions
| Problem Category | Specific Cause | Recommended Solution |
|---|---|---|
| Probe Penetration | Impermeable cell walls (especially in Gram-positive bacteria) leading to poor probe delivery [17] | Optimize fixation and permeabilization; Use chemical transformation (e.g., CaCl2 heat shock) for living cells [17] |
| Target Accessibility | Low cellular rRNA content in slow-growing environmental bacteria [58] | Use more sensitive probes (e.g., Molecular Beacons); Increase probe concentration [58] |
| Detection Sensitivity | High background fluorescence obscuring specific signal [58] | Switch to self-quenching probes (e.g., Molecular Beacons); Optimize post-hybridization washes [58] |
Detailed Experimental Protocols for Signal Optimization
Protocol 1: Standard FISH Optimization with Molecular Beacons
Molecular beacons are self-reporting probes that fluoresce only upon hybridization, significantly reducing background noise and improving the signal-to-noise ratio (S:N) [58]. This protocol is adapted for the detection of Pseudomonas putida but can be modified for other targets.
Probe Design:
- Design a molecular beacon probe targeting the 16S rRNA of your specific microbe. The probe sequence should be flanked by inverted repeats that form stem-loop structures.
- Example: The Ps440MB beacon sequence for pseudomonads is 5′-ACGGGCCCTTCCTCCCAACTTCCCGT-3′ (stems in bold) [58].
- Label the 5′ end with a fluorophore (e.g., 6-carboxyfluorescein) and the 3′ end with a quencher (e.g., DABCYL).
Cell Fixation:
- Fix cells from laboratory cultures with 3 volumes of 4% fresh paraformaldehyde in phosphate-buffered saline (PBS, pH 8.0) for 1 hour at room temperature [58].
- Wash cells with PBS and resuspend in a 1:1 (vol/vol) mixture of PBS and absolute ethanol. Fixed cells can be stored at -20°C.
Hybridization:
- Add approximately 2 × 105 fixed cells to 50 µL of hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl pH 7.2, 0.1% SDS).
- Add the molecular beacon probe to a final concentration of 0.1 µM.
- Incubate the hybridization mixture for 1 hour at 55°C [58].
Analysis:
- Analyze samples using flow cytometry or fluorescence microscopy. Post-hybridization washing is often unnecessary with molecular beacons due to their low background and can lead to cell loss [58].
Protocol 2: live-FISH for Signal Detection in Living Bacteria
This fixation-free method (live-FISH) is designed to hybridize probes to living bacterial cells, which can sometimes yield a better signal than in fixed cells [17].
Cell Preparation:
- Grow bacterial cultures to late logarithmic growth phase. Wash cells three times with 1x PBS, avoiding ethanol [17].
Pre-hybridization and Probe Delivery:
- Resuspend the washed cell pellet in 50 µL of 100 mM CaCl2.
- Add the fluorescently labelled DNA probe (e.g., 4 ng/µL) and incubate for 15 minutes on ice.
- Perform a heat shock by incubating the mixture at 42°C for 60 seconds, then return it briefly to ice [17].
Hybridization:
- Immediately add 500 µL of pre-warmed hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl pH 7.4, 0.01% SDS, 35% formamide) at 46°C.
- Hybridize by incubating for 2 hours at 46°C with shaking [17].
Washing:
- Pellet cells at 10,000 × g for 5 minutes.
- Resuspend in 500 µL of pre-warmed wash buffer (20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS, 0.080 M NaCl) and incubate at 48°C for 20 minutes.
- Centrifuge twice in 500 µL of ice-cold 1x PBS and keep in this buffer on ice for analysis [17].
The following workflow diagram illustrates the key decision points and procedural steps for the two optimization strategies.
Quantitative Data for Method Selection
The choice of probe and protocol has a significant, measurable impact on experimental outcomes. The data below quantitatively compare the performance of molecular beacons versus linear probes.
Table 2: Quantitative Comparison of FISH Probe Performance in Pure Culture [58]
| Probe Type | Target Organism | Non-Target Organism | Mean Fluorescent Intensity (MFI) Target | Mean Fluorescent Intensity (MFI) Non-Target | Signal-to-Noise (S:N) Ratio |
|---|---|---|---|---|---|
| Molecular Beacon (Ps440MB) | Pseudomonas putida | Escherichia coli | 133.4 | 9.4 | 14.2 |
| Linear Probe (Ps440LP) | Pseudomonas putida | Escherichia coli | 147.3 | 18.7 | 7.9 |
Table 3: Efficacy of live-FISH Protocol on Cell Viability and Detection [17]
| Protocol Step | Buffer/Technique | Impact on Cell Viability (CFU) | Key Function |
|---|---|---|---|
| Pre-hybridization | PBS or Artificial Seawayer (ASW) | Maintains viability | Preserves living cells for downstream cultivation |
| Probe Delivery | Chemical transformation (CaCl2) with heat shock | Maintains viability | Introduces labelled DNA probes without fixation |
| Hybridization | Buffer with formamide and low SDS (0.01%) | Maintains viability | Enables specific probe-target binding in living cells |
The Scientist's Toolkit: Research Reagent Solutions
A successful FISH experiment relies on a set of key reagents, each fulfilling a specific role in ensuring signal specificity and intensity.
Table 4: Essential Reagents for FISH Troubleshooting
| Reagent / Material | Function / Purpose | Example / Note |
|---|---|---|
| Molecular Beacon Probes | Self-quenching probes that reduce background noise and increase signal-to-noise ratio by fluorescing only upon binding to the target sequence [58]. | Ps440MB for pseudomonads; Superior to linear probes for discriminating target cells in complex samples [58]. |
| Paraformaldehyde (PFA) | Fixative that preserves cellular morphology and immobilizes target nucleic acids within the cell. Essential for standard FISH protocols [58]. | Typically used at 4% concentration; fixation time must be optimized to balance morphology preservation with probe accessibility. |
| Peptide Nucleic Acid (PNA) Probes | Synthetic DNA analogs with a neutral backbone that improve hybridization efficiency and cell permeability, often used for difficult targets [8]. | Mentioned as a viable probe strategy; Can be used in PNA-based molecular beacons [58]. |
| Formamide | Denaturing agent added to the hybridization buffer to control the stringency of hybridization, ensuring specific binding of the probe to its target [17]. | Concentration (e.g., 35%) is varied to fine-tune hybridization specificity and prevent off-target binding [17]. |
| Calcium Chloride (CaCl2) | Used in chemical transformation to facilitate the delivery of DNA probes through the cell membranes of living bacteria in live-FISH protocols [17]. | A critical component of the pre-hybridization buffer in the live-FISH protocol [17]. |
| Tridecaptin A(sup alpha) | Tridecaptin A(sup alpha), CAS:67922-28-5, MF:C73H115N17O20, MW:1550.8 g/mol | Chemical Reagent |
| Dirhodium trisulphite | Dirhodium trisulphite, CAS:80048-77-7, MF:O9Rh2S3, MW:446.0 g/mol | Chemical Reagent |
Strategies for Reducing High Background and Non-Specific Hybridization
In the field of microbial detection research, fluorescence in situ hybridization (FISH) is a powerful technique that allows for the direct visualization of specific microbial populations within their environmental context. However, the effectiveness of this method is often compromised by high background fluorescence and non-specific hybridization, which can obscure critical data and lead to erroneous conclusions. These challenges are particularly pronounced in complex environmental samples such as subseafloor sediments, which contain inorganic mineral grains that readily adsorb fluorescent probes [59]. This application note provides a comprehensive framework of strategies and detailed protocols to overcome these limitations, enabling researchers to achieve the high signal-to-noise ratios necessary for reliable microbial detection and quantification.
Background interference in FISH assays originates from multiple technical aspects of the procedure. Non-specific hybridization occurs when probes bind to non-target sequences or cellular components, while high background fluorescence can stem from factors such as autofluorescence of biological samples, insufficient washing, or probe adsorption to non-target surfaces [60] [59]. In microbial ecology, a significant challenge arises from the non-specific adsorption of fluorescent oligonucleotide probes onto mineral surfaces present in environmental samples like sediments. This adsorption creates bright fluorescence on mineral particles that can completely compromise the specific detection of active microbial populations [59]. Furthermore, suboptimal sample preparation, including improper fixation and pre-treatment, can exacerbate these issues by either exposing non-specific binding sites or damaging target sequences [60].
Table: Common Sources of High Background in FISH Assays and Their Characteristics
| Source Category | Specific Source | Manifestation | Primary Affected Samples |
|---|---|---|---|
| Sample-Related | Autofluorescence | Uniform background signal | All biological samples, particularly tissues |
| Mineral particle adsorption | Bright, speckled fluorescence on particles | Environmental samples (sediments, soils) | |
| Incomplete fixation | Diffuse non-specific signal | FFPE tissues, cell smears | |
| Probe-Related | Repeated sequences in probes | Off-target binding to similar sequences | All sample types |
| Excessive probe concentration | High uniform background | All sample types | |
| Non-optimized denaturation | Either weak signals or high background | FFPE tissues | |
| Protocol-Related | Insufficient washing | High uniform background | All sample types |
| Inadequate stringency | Non-specific binding retained | All sample types | |
| Degraded buffers | Increased background fluorescence | All sample types |
Strategic Approaches for Background Reduction
Sample Preparation and Pre-Treatment Optimization
Proper sample preparation establishes the foundation for high-quality FISH results with minimal background. Fixation represents one of the most critical steps, particularly for formalin-fixed paraffin-embedded (FFPE) samples, where both under-fixation and over-fixation can lead to elevated background signals. Under-fixation results in incomplete preservation of cellular structure, increasing the risk of DNA degradation and non-specific probe binding. Conversely, over-fixation promotes excessive cross-linking of proteins and nucleic acids, which can mask target sequences and increase background through non-specific binding [60].
For optimal results:
- Use freshly prepared fixative solutions and adhere strictly to recommended fixation times
- For blood smear slides, consider using hypotonic solutions such as potassium chloride during fixation to reduce background fluorescence
- For FFPE tissues, aim for sections of 3-4μm thickness to avoid issues with probe penetration and interpretation [60]
Pre-treatment steps designed to break down proteins, lipids, and other cellular components that may mask target DNA sequences must be carefully balanced. Insufficient pre-treatment may leave behind cellular debris that exerts natural autofluorescence or acts as non-specific binding sites. Over-digestion, however, may damage your sample and target sequence, resulting in low signal intensity [60]. For FFPE tissues, specialized pretreatment kits such as the CytoCell LPS 100 Tissue Pretreatment Kit can provide optimized conditions when used according to manufacturer specifications, including heating pretreatment solution to 98–100°C before enzyme treatment at 37°C [60].
Probe Design and Hybridization Optimization
Probe design profoundly influences hybridization specificity. A fundamental principle is avoiding short repeated sequences (k-mers) within probes, as even 20 nt perfect repeated sequences within much longer probes (e.g., 350–1500 nt) can produce significant off-target signals. Research demonstrates that removing these small regions of repeated sequence can increase the signal-to-noise ratio by orders of magnitude, enabling quantitative measurement of target transcript numbers [61]. Computational tools are available to annotate k-mer uniqueness across genomes, allowing researchers to design probes lacking these problematic repeats [61].
Hybridization conditions must be meticulously controlled:
- Probe volume must be optimized—too little results in weak signals, while excess leads to high background [60]
- Denaturation temperature and time require precise optimization, particularly for FFPE samples with extensive cross-linking [60]
- Hybridization buffer composition can be modified to reduce background, such as incorporating EDTA to prevent non-specific adsorption [59]
For environmental samples rich in mineral particles, the novel EDTA-FISH approach provides a significant advancement. By replacing 0.9 M NaCl with 250 mM EDTA in the hybridization buffer, this method effectively reduces non-specific adsorption of probes to mineral surfaces while maintaining hybridization efficiency to target microbial cells [59].
Table: Quantitative Optimization Parameters for FISH Hybridization and Washing
| Parameter | Sub-Optimal Condition | Consequence | Optimal Range | Effect on Background |
|---|---|---|---|---|
| Formamide Concentration | Incorrect for specific probe | Reduced specificity or signal loss | Probe-specific (typically 5-35%) | Critical - requires probe-specific optimization [59] |
| Hybridization Temperature | Too low or too high | Non-specific binding or reduced hybridization | Typically 46°C for EDTA-FISH [59] | High temperature decreases background but may reduce signal |
| Hybridization Time | Too prolonged | Increased off-target binding | 2-3 hours [59] | Extended time increases background |
| Wash Stringency (SSC concentration) | Too low | Failure to remove non-specifically bound probes | Varies with protocol | Lower salt increases stringency, decreases background [60] |
| Wash Temperature | Too low | Inadequate removal of non-specific binding | Typically 48°C for EDTA-FISH [59] | Higher temperature increases stringency |
| EDTA Concentration in Buffer | None in mineral-rich samples | High non-specific adsorption | 250 mM for problematic samples [59] | Dramatically reduces mineral adsorption |
Post-Hybridization Washes and Detection
Stringent washing after hybridization is crucial for removing excess unbound or non-specifically bound probes without disrupting specific probe-target hybrids. The stringency of washes can be optimized by carefully adjusting incubation pH, temperature, and time. Begin with the recommended parameters in your protocol and make incremental adjustments if background persists [60]. Always use freshly prepared wash buffers to prevent contamination or degradation that could contribute to background fluorescence [60].
For challenging samples with persistent background, enzymatic treatments can be employed after hybridization:
- RNase A treatment (50 µg/mL for 30-60 minutes at 37°C) prior to hybridization confirms RNA-specific signals [62]
- S1 nuclease (single-strand-specific endonuclease) for DNA probes and RNase A for RNA probes can digest non-specifically bound probes before detection [63]
When using signal amplification methods such as tyramide signal amplification (TSA), ensure that the amplification reagents are fresh and properly diluted, as over-amplification can increase background signals non-specifically. Newer signal amplification technologies like HCR (hybridization chain reaction) offer more linear amplification with potentially lower background [64].
EDTA-FISH: A Specialized Protocol for Environmental Samples
The EDTA-FISH protocol represents a significant innovation for reducing background in mineral-rich environmental samples. This method specifically addresses the challenge of probe adsorption to mineral surfaces by incorporating a high concentration of EDTA in the hybridization buffer [59].
Principle
EDTA (ethylenediaminetetraacetic acid) is a chelating agent that prevents the adsorption of oligonucleotide probes to mineral particles by chelating divalent cations that would otherwise facilitate DNA binding to mineral surfaces. This approach dramatically reduces non-specific background while maintaining specific hybridization to target microbial cells [59].
Materials and Reagents
- Hybridization buffer with EDTA: 250 mM EDTA (pH 8.0), 20 mM Tris/HCl, 0.01% SDS, and appropriate formamide concentration (5-35% based on probe requirements)
- Note: NaCl is omitted from the standard FISH buffer composition to avoid high Na+ concentrations
- Washing buffer: Appropriate stringency buffer based on probe requirements
- Standard FISH reagents for sample preparation, fixation, and mounting
Step-by-Step Protocol
- Sample Preparation: Fix samples with paraformaldehyde (e.g., 4% in PBS for 2-4 hours), wash with PBS, and preserve in EtOH/PBS (1:1) at -20°C until use.
- Slide Preparation: Place sediment slurry or sample on glass slide, embed in low gelling point agarose (0.1%), and dehydrate through an ethanol series (50%, 80%, and 99.5% ethanol), then air-dry.
- Hybridization:
- Prepare hybridization buffer with 250 mM EDTA and appropriate formamide concentration
- Apply probe in EDTA-containing hybridization buffer to samples
- Incubate at 46°C for 2 hours in a humidified chamber
- Washing:
- Remove excess probe by washing in appropriate buffer at 48°C for 20 minutes
- Perform additional washes as needed based on specific probe requirements
- Detection and Imaging:
- Mount samples with appropriate anti-fade mounting medium
- Image using epifluorescence or confocal microscopy with appropriate filters
Critical Notes
- The optimal formamide concentration needs to be determined probe-by-probe as the dissociation curves may shift to lower formamide concentrations compared to standard FISH [59]
- EDTA-FISH maintains the capacity for mismatch discrimination, though single mismatch discrimination may require competitor probes as in standard FISH [59]
- This method is compatible with various FISH probe types and can be adapted to more advanced FISH protocols such as hybridization chain reaction-FISH [59]
Essential Controls for Background Assessment
Implementing appropriate controls is essential for distinguishing specific signals from background and validating FISH results in microbial detection research.
Critical Control Experiments
- RNase pretreatment control: Treat samples with RNase A (50 µg/mL) for 30-60 minutes at 37°C prior to hybridization. Specific RNA signals should disappear with RNase treatment, confirming the signal originates from RNA and not other sources [62].
- No-probe control: Process samples with hybridization buffer only (no probe) to identify autofluorescence and non-specific binding of detection reagents [62].
- Filter control: Image samples in an unused filter (one that your fluorophore should not be detected in) to identify autofluorescent particles that might be mistaken for specific signals [62].
- Negative biological control: Use samples (cell lines or tissues) void of the target transcript to confirm probe specificity [62].
- Positive control: Use validated, catalogued probe sets targeting genes known to be expressed in your sample to confirm technical success [62].
Research Reagent Solutions
Table: Essential Reagents for Background Reduction in FISH Assays
| Reagent/Category | Specific Examples | Function in Background Reduction | Application Context |
|---|---|---|---|
| Specialized Buffers | EDTA-containing hybridization buffer [59] | Reduces non-specific adsorption to mineral particles | Environmental samples (sediments, soils) |
| Freshly prepared wash buffers [60] | Effectively removes non-specifically bound probes | All FISH applications | |
| Pretreatment Kits | CytoCell LPS 100 Tissue Pretreatment Kit [60] | Optimized enzyme digestion to reduce masking of targets | FFPE tissue samples |
| Probe Technologies | HCR (Hybridization Chain Reaction) probes [64] | Linear amplification with low background | Sensitive RNA detection in thick tissues |
| ECHO-FISH probes [33] | Hybridization-sensitive activation minimizes washing needs | Fast FISH protocols | |
| Stellaris RNA FISH probes [62] | Designed for minimal off-target hybridization | Quantitative RNA detection | |
| Signal Amplification | Tyramide SuperBoost Kits [65] | Controlled amplification reduces non-specific background | Low-abundance targets |
| Enzymatic Treatments | RNase A [62] | Removes RNA-specific background | Verification of RNA signals |
| Proteinase K [63] | Optimized digestion improves target accessibility | All tissue-based FISH | |
| Microscopy Components | Quality optical filters [60] | Prevents signal attenuation and distortion | All fluorescence microscopy |
Reducing high background and non-specific hybridization in FISH assays requires a systematic approach addressing sample preparation, probe design, hybridization conditions, and detection parameters. The implementation of the EDTA-FISH protocol for mineral-rich environmental samples represents a significant advancement for microbial detection research, effectively overcoming the challenge of probe adsorption to mineral surfaces. Similarly, careful attention to fixation conditions, pre-treatment optimization, and stringent washing procedures can dramatically improve signal-to-noise ratios across various sample types. By incorporating appropriate controls and considering advanced probe technologies, researchers can achieve the specificity and sensitivity required for accurate microbial detection and quantification. These strategies collectively enhance the reliability of FISH as a powerful tool for investigating microbial ecology in complex environmental samples.
Addressing Weak, Faded, or Uneven Signal Distribution
Weak, faded, or uneven signal distribution presents a significant challenge in Fluorescence in Situ Hybridization (FISH), potentially compromising data accuracy in microbial detection research. These issues can stem from various factors including poor probe penetration, low hybridization efficiency, target inaccessibility, and fluorophore decay. This application note details standardized protocols and advanced methodologies to mitigate these challenges, ensuring robust and reproducible signal detection. The focus is on microbial research applications where signal optimization is crucial for reliable analysis of microbial community structure, function, and spatial organization.
The table below summarizes performance metrics for various FISH signal enhancement and stabilization strategies, providing a comparative overview for method selection.
Table 1: Quantitative Performance Metrics of FISH Signal Enhancement Methods
| Method | Key Mechanism | Signal Enhancement Factor | Processing Time | Best for Microbial Targets | Key Limitations |
|---|---|---|---|---|---|
| TDDN-FISH [66] | Enzyme-free, dendritic DNA nanostructure | Significantly stronger than smFISH & HCR-FISH [66] | ~1 hour per round [66] | Short RNAs, low-abundance transcripts | Complex probe design and assembly |
| Live-FISH (with PMA treatment) [5] | Viability staining to reduce background | N/A (Viability context specific) | Varies with sample | Planctomycetota, Bacillota [5] | Reduces total viable cells; taxon-specific viability effects [5] |
| TrueProbes Computational Design [39] | Genome-wide BLAST & thermodynamic modeling | Enhances signal-to-noise by minimizing off-target binding [39] | Computational time | All targets, especially genes with shared motifs | Requires bioinformatics expertise |
| Conventional smFISH | Multiple (~48) labeled probes per target | Baseline | ~1 hour [66] | Highly expressed genes | Constrained by target mRNA length [66] |
Detailed Experimental Protocols
Protocol: Tetrahedral DNA Dendritic Nanostructure-Enhanced FISH (TDDN-FISH)
This enzyme-free protocol uses self-assembling DNA nanostructures for rapid, high-intensity signal amplification, ideal for detecting short RNA targets or low-abundance transcripts [66].
Reagents and Materials
- Tetrahedral DNA Monomers (T0, T1, T2): Self-assembled from four complementary oligonucleotide strands (17 bp side length) [66].
- Bifunctional Primary Probe: Contains a target-specific sequence and a readout sequence for TDDN attachment [66].
- Fluorophore-labeled Oligonucleotides: Complementary to T2 monomer sticky ends.
- Hybridization Buffer: With optimized formamide concentration (10-30%) [66].
- Mounting Medium with antifade agents.
Step-by-Step Procedure
- Sample Preparation: Fix microbial cells according to standard protocols (e.g., 4% paraformaldehyde). Permeabilize cells to facilitate probe access.
- Primary Probe Hybridization:
- Apply the bifunctional primary probe to the sample.
- Incubate at 37–42°C for a duration optimized for your target and probe. This temperature range was systematically optimized for TDDN-FISH [66].
- TDDN Assembly and Hybridization:
- Washing:
- Perform post-hybridization washes with a saline-sodium citrate (SSC) buffer to remove unbound probes and nanostructures, minimizing background.
- Imaging:
- Mount samples and image using a fluorescence or confocal microscope. TDDN-FISH's strong signal enables low-magnification imaging while maintaining subcellular resolution [66].
Protocol: Viability-Linked Live-FISH for Soil Microbiomes
This protocol combines fluorescence in situ hybridization with viability staining to target metabolically active microbes in complex environmental samples like soil [5].
Reagents and Materials
- Specific Oligonucleotide Probes: 16S rRNA-targeting probes labeled with fluorophores (e.g., CY3).
- Propidium Monoazide (PMA): Viability dye for suppressing signal from compromised cells.
- Live-FISH Hybridization Buffer.
- Flow Cytometry Buffer (e.g., 1x PBS).
Step-by-Step Procedure
- Sample Pre-treatment:
- Extract microorganisms from the soil matrix using gentle dispersion in a suitable buffer (e.g., 1x PBS).
- Live-FISH Hybridization:
- Resuspend the extracted cells in Live-FISH hybridization buffer containing the fluorescently labeled probe.
- Incubate in the dark at the appropriate hybridization temperature (e.g., 46°C for 2-3 hours).
- PMA Treatment:
- Treat hybridized cells with PMA (e.g., 50 µM final concentration) and incubate for 10 minutes in the dark.
- Photo-activate the PMA dye by exposing the sample to bright light (e.g., a 650 W halogen lamp for 5 minutes).
- Cell Sorting and Analysis:
- Analyze and sort the labeled, viable cells using a fluorescence-activated cell sorter (FACS).
- Gate the population based on forward/side scatter and fluorescence intensity to select the target taxa.
Troubleshooting Notes
- Taxon-Specific Viability: Note that Live-FISH treatment has taxon-specific effects on viability. For example, viability of Planctomycetota and Bacillota is retained better than that of Acidobacteriota, which can be reduced by several orders of magnitude [5].
- Signal Optimization: If the fluorescence signal is weak, optimize the probe concentration and hybridization time. Ensure the PMA light exposure step is correctly calibrated to avoid non-specific signal loss.
Workflow: High-Fidelity Probe Design with TrueProbes
Computational probe design is critical for maximizing on-target binding and minimizing off-target background, which directly addresses uneven and faded signals [39].
Diagram 1: TrueProbes computational design workflow for high-specificity FISH probes. [39]
- Input and Tile: Input the target RNA sequence and tile it with all oligonucleotides of a defined length (e.g., 17-22mer) [39].
- Off-Target Analysis: Use BLAST to identify and quantify potential off-target binding across the entire genome or transcriptome [39].
- rRNA Filtering: Remove any probe sequences with significant complementarity to ribosomal RNA (rRNA) genes to reduce background [39].
- Thermodynamic Scoring: Calculate the Gibbs free energy (ΔG°) for on-target and off-target binding for each probe [39].
- Ranking and Selection: Rank all candidate probes from highest to lowest predicted specificity, considering minimal expressed off-target binding and strong on-target affinity. Iteratively select the top-ranked, non-overlapping probes to form the final set [39].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagent Solutions for Optimizing FISH Signals
| Reagent / Material | Function | Application Notes |
|---|---|---|
| Tetrahedral DNA Dendritic Nanostructures (TDDNs) | Enzyme-free signal amplification structure. Exponential signal multiplication via hierarchical DNA assembly. [66] | Ideal for rapid detection of short RNAs and low-abundance targets. Requires careful assembly and validation. |
| Propidium Monoazide (PMA) | Viability dye. Penetrates compromised cells, binds DNA, and suppresses PCR/background signals after light activation. [5] | Critical for Live-FISH to distinguish intact, viable cells in complex microbiomes (e.g., soil). |
| High-Specificity FISH Probes | Target-specific oligonucleotides for hybridization. | Using computationally designed probes (e.g., via TrueProbes) minimizes off-target binding, a primary cause of high background and uneven signal. [39] |
| Formamide-Based Hybridization Buffers | Denaturing agent in hybridization buffer. Reduces non-specific binding and lowers effective hybridization temperature. | Concentration (10-30%) must be optimized for each probe and target to balance signal strength and specificity. [66] |
| Henicosyl methacrylate | Henicosyl methacrylate, CAS:45296-31-9, MF:C25H48O2, MW:380.6 g/mol | Chemical Reagent |
Addressing weak or uneven signals in FISH requires a multifaceted strategy combining advanced probe design, innovative signal amplification techniques, and sample-specific protocol optimization. The methods detailed here—TDDN-FISH for robust amplification, Live-FISH for viability-linked detection in complex samples, and TrueProbes for superior probe specificity—provide researchers with a powerful toolkit to overcome critical sensitivity and distribution challenges. By systematically implementing these protocols, scientists can achieve highly reliable, quantitative, and spatially accurate detection of microbial targets, thereby advancing research in microbial ecology, diagnostics, and drug development.
Optimizing Permeabilization and Denaturation for Intact Cell Morphology
In fluorescence in situ hybridization (FISH), the dual objectives of achieving sufficient probe penetration while preserving pristine cell morphology present a significant technical challenge. Permeabilization and denaturation conditions must be carefully balanced to allow nucleic acid probes access to their intracellular targets without compromising structural integrity. This balance is particularly crucial in microbial detection research and drug development, where accurate cellular localization and quantification are paramount. This application note systematizes optimized protocols for permeabilization and denaturation, drawing on recent methodological advances to guide researchers in selecting and implementing the most effective procedures for their experimental contexts.
Quantitative Comparison of Permeabilization and Denaturation Methods
The selection of permeabilization method significantly impacts the success of FISH experiments. The table below summarizes the performance characteristics of various techniques, providing a basis for evidence-based protocol selection.
Table 1: Performance Characteristics of FISH Permeabilization Methods
| Method | Optimal Concentration/Duration | Target Applications | Morphology Preservation | Signal Strength | Key Advantages |
|---|---|---|---|---|---|
| Proteinase K | 50 μg/ml for 1 hour [67] | RNA ISH, RNA FISH on Drosophila ovaries [67] | Good with post-fixation [67] | Strong colorimetric signal [67] | Highly effective for difficult tissues [67] |
| Detergents (RIPA) | Not specified | IF/FISH on Drosophila ovaries [67] | Good with combined methods [67] | Moderate alone, enhanced in combination [67] | Preserves protein epitopes for IF/FISH [67] |
| Organic Solvents (Xylenes) | Not specified | IF/FISH on Drosophila ovaries [67] | Good with combined methods [67] | Variable alone, enhanced in combination [67] | Preserves protein epitopes for IF/FISH [67] |
| Combined RIPA + Xylenes | Sequential application | IF/FISH on Drosophila ovaries [67] | Good [67] | Strongest non-proteinase K option [67] | Optimal balance for protein and RNA detection [67] |
| Ethanol Series | Gradual concentration changes | Live-FISH on bacteria [17] | Excellent (maintains viability) [17] | Sufficient for identification [17] | Maintains cell viability; enables live cell sorting [17] |
| Chemical Transformation (CaClâ‚‚) | 100 mM CaClâ‚‚, 15 min on ice [17] | Live-FISH on bacteria [17] | Excellent (maintains viability) [17] | Sufficient for identification [17] | Maintains cell viability; enables culture of sorted cells [17] |
The effectiveness of denaturation conditions is equally critical and varies significantly based on probe characteristics. Systematic optimization is essential for achieving optimal signal-to-noise ratios.
Table 2: Denaturation and Hybridization Optimization Parameters
| Parameter | Tested Conditions | Optimal Values | Experimental System | Impact on Performance |
|---|---|---|---|---|
| Formamide Concentration | Varied concentrations with fixed 37°C temperature [40] | Depends on probe length [40] | MERFISH with 20-50 nt target regions [40] | Weak dependence within optimal range; critical for specificity [40] |
| Target Region Length | 20, 30, 40, 50 nt [40] | All lengths performed well with optimized formamide [40] | smFISH on U-2 OS cells [40] | Brightness depends weakly on length with proper optimization [40] |
| Hybridization Time | Up to 5 hours [58] | 1 hour for DNA molecular beacons [58] | Flow-FISH for Pseudomonas putida [58] | Extended times do not necessarily improve signal [58] |
| Hybridization Temperature | Room temperature to 65°C [58] | 55°C for DNA molecular beacons [58] | Flow-FISH for bacterial detection [58] | Higher temperatures improve specificity [58] |
Experimental Protocols for Optimized FISH
Protocol 1: Proteinase K-Based Permeabilization for Standard FISH
This protocol is optimized for challenging tissues where maximum probe penetration is required and viability maintenance is not necessary [67].
Materials:
- Proteinase K (50 μg/ml in appropriate buffer)
- PBS or alternative physiological buffer
- 4% paraformaldehyde with 1% DMSO
- Ethanol series (30%, 50%, 70%, 80%, 95%, 100%)
Procedure:
- Fix dissected tissue samples in 4% paraformaldehyde with 1% DMSO for 1 hour at room temperature.
- Dehydrate samples through a graded ethanol series (30%, 50%, 70%, 80%, 95%, 100%).
- Rehydrate through a reverse ethanol series to aqueous buffer.
- Treat with proteinase K (50 μg/ml) for 1 hour at room temperature.
- Post-fix in 4% paraformaldehyde for 30 minutes to restore morphology.
- Proceed with standard FISH hybridization protocol.
Protocol 2: Viability-Preserving Live-FISH for Bacteria
This protocol enables FISH labeling while maintaining bacterial viability for subsequent cultivation, using chemical transformation rather than destructive permeabilization [17].
Materials:
- Calcium chloride (100 mM)
- Hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl pH 7.4, 0.01% SDS, 35% formamide)
- Wash buffer (20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS, 0.080M NaCl)
- Marine Broth or appropriate growth medium
Procedure:
- Grow bacterial cultures to late logarithmic phase (OD₆₀₀ₙₘ = 0.5-0.8).
- Wash cells three times with 1× PBS by gentle centrifugation.
- Resuspend washed cells in 50 μl of 100 mM CaCl₂.
- Add fluorescent probe (4 ng/μl final concentration) and incubate on ice for 15 minutes.
- Apply heat shock at 42°C for 60 seconds, then return to ice briefly.
- Immediately add 500 μl pre-warmed hybridization buffer (46°C) and hybridize for 2 hours at 46°C.
- Pellet cells and wash twice with pre-warmed wash buffer (48°C).
- Resuspend in ice-cold PBS for immediate analysis or sorting.
Protocol 3: Dual Protein-RNA Detection (IF/FISH)
This protocol optimizes conditions for simultaneous detection of protein and RNA targets by reversing the traditional order of operations and using alternative permeabilization [67].
Materials:
- RIPA buffer
- Xylenes
- Primary and secondary antibodies for target proteins
- RNA FISH probes
- 4% paraformaldehyde
Procedure:
- Fix tissues briefly (20 minutes) in 4% paraformaldehyde.
- Perform complete immunofluorescence staining using standard protocols.
- Post-fix in 4% paraformaldehyde for 30 minutes to cross-link antibodies.
- Permeabilize sequentially with xylenes and RIPA buffer.
- Perform FISH hybridization without proteinase K treatment.
- Wash and mount for imaging.
Method Selection Workflow
The following diagram illustrates the decision process for selecting the optimal permeabilization strategy based on experimental requirements:
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for FISH Permeabilization and Denaturation Optimization
| Reagent/Category | Specific Examples | Function in FISH | Application Notes |
|---|---|---|---|
| Enzymatic Permeabilization | Proteinase K [67] | Digests structural proteins for probe access | Use at 50 μg/ml for 1h with post-fixation; optimal for difficult tissues [67] |
| Detergent-Based Permeabilization | RIPA Buffer, SDS [67] | Dissolves lipid membranes | Preserves protein epitopes for IF/FISH; use in combination with organic solvents [67] |
| Organic Solvent Permeabilization | Xylenes, Ethanol series [17] [67] | Extracts lipids and dehydrates tissue | Ethanol series maintains viability in Live-FISH; xylenes preserves protein antigens [17] [67] |
| Chemical Transformation Agents | Calcium chloride [17] | Facilitates DNA probe uptake in live cells | 100mM concentration with heat shock enables viability-preserving Live-FISH [17] |
| Denaturation Agents | Formamide [40] | Lowers melting temperature of nucleic acids | Concentration must be optimized for specific probe length and hybridization temperature [40] |
| Hybridization Buffers | Salt-based buffers with denaturants [17] | Provides optimal ionic and denaturing conditions | Typical composition: 0.9M NaCl, 20mM Tris-HCl, 0.01% SDS, 35% formamide [17] |
Successful FISH imaging depends critically on the careful optimization of permeabilization and denaturation conditions. While proteinase K treatment remains the gold standard for challenging tissues where maximum penetration is required, alternative methods such as detergent-organic solvent combinations better preserve protein epitopes for simultaneous protein-RNA detection. Most notably, viability-preserving Live-FISH protocols enable entirely new applications where maintenance of cellular function is essential. By systematically applying the optimized protocols and selection framework presented here, researchers can significantly enhance the quality and reliability of their FISH-based microbial detection assays.
Probe quality control is a foundational element in obtaining reliable and reproducible results in Fluorescence In Situ Hybridization (FISH) for microbial detection. The effectiveness of a FISH experiment, which relies on fluorescently labeled nucleic acid probes binding to specific target sequences within cells, is directly contingent on the labeling efficiency and purity of the probes used. Poorly labeled or impure probes can lead to weak signals, high background noise, and false-negative or false-positive results, ultimately compromising data integrity. This application note details standardized protocols and quantitative assessments to ensure that FISH probes meet the stringent quality standards required for advanced microbial research and drug development.
Probe Design and Synthesis
The journey to high-quality FISH results begins with meticulous probe design and synthesis. For microbial detection, probes are typically short oligonucleotides (20-25 nucleotides) targeting specific regions of ribosomal RNA (rRNA), which is abundant in bacterial cells, thereby amplifying the signal. The design process must ensure target specificity to avoid cross-hybridization with non-target sequences. Probes are often designed using specialized software and must be checked against genomic databases.
Following design, probes are synthesized. A common and effective method is in vitro transcription from cloned DNA templates. This process involves using RNA polymerases (T7, T3, or SP6) to incorporate labeled nucleotides into the nascent RNA strand. As described in a protocol for marine organisms, probes can be synthesized from "linearized, cloned and amplified DNA fragments," which serve as templates for the transcription reaction. Labeling can be performed during transcription by incorporating modified nucleotides (e.g., digoxigenin-, fluorescein-, or DNP-labeled UTP) or post-transcriptionally through chemical labeling [68]. Alternatively, DNA oligonucleotide probes can be chemically synthesized and subsequently labeled via enzymatic reactions or direct chemical modification.
Quantitative Quality Control Assessment
After synthesis, probes must undergo rigorous quantitative assessment. The following parameters are critical for determining probe suitability and ensuring experimental consistency. The table below summarizes the key quality control metrics, their ideal values, and the techniques used for measurement.
Table 1: Key Quality Control Metrics for FISH Probes
| Quality Parameter | Description | Target Value/Range | Assessment Method |
|---|---|---|---|
| Labeling Efficiency | Moles of label incorporated per mole of nucleic acid. | Varies by label; sufficient for clear signal detection. | Spectrophotometry (Absorbance), Fluorometry |
| Probe Purity | Absence of truncated or degraded nucleic acid fragments and unincorporated labels. | Distinct single band on gel. | Agarose or Polyacrylamide Gel Electrophoresis |
| Concentration | Amount of probe nucleic acid present in solution. | Sufficient for hybridization (e.g., 5-10 ng/μL). | Spectrophotometry (A260) |
| Functional Validation | Specific binding to target sequence with minimal non-specific background. | Strong signal at target, low background. | Dot-Blot Assay, FISH on control samples |
Spectrophotometric and Fluorometric Analysis
- Procedure: Dilute the probe in an appropriate buffer. Measure absorbance from 220 nm to 700 nm using a spectrophotometer.
- Data Interpretation: Nucleic acid concentration is calculated from the absorbance at 260 nm (A260). Contamination is assessed by examining ratios (e.g., A260/A280 ~1.8-2.0 for pure RNA/DNA; A260/A230 >2.0 for low organic contaminant levels). The concentration of the incorporated label (e.g., fluorescein, digoxigenin) can often be determined from its specific absorbance peak, allowing for an estimation of labeling efficiency [8].
- Advanced Technique: For fluorescent labels, fluorometry provides a more sensitive and specific measurement of label concentration, which can be correlated with nucleic acid concentration to calculate a precise labeling ratio.
Gel Electrophoretic Analysis
- Procedure: Analyze an aliquot of the probe (e.g., 100-200 ng) on a denaturing agarose or polyacrylamide gel alongside appropriate molecular weight markers and a negative control (transcription reaction without template).
- Data Interpretation: A single, sharp band of the expected size indicates high probe purity. A smeared appearance suggests degradation, while multiple discrete bands may indicate incomplete transcription or degradation. The absence of a smeared background at the dye front confirms that unincorporated nucleotides have been successfully removed during purification [69].
Functional Validation via Dot-Blot Assay
- Procedure: Spot a dilution series of both the target DNA and a non-target control DNA onto a nitrocellulose or nylon membrane. Fix the DNA to the membrane. Hybridize the membrane with the labeled probe under conditions similar to those used in FISH (e.g., appropriate temperature and formamide concentration). Perform stringent washes and detect the signal using the intended detection system (e.g., enzyme-conjugated antibodies for digoxigenin followed by a colorimetric substrate).
- Data Interpretation: A strong signal from the target DNA spots and no signal from the non-target control indicates high specificity and functional activity of the probe. This assay provides a direct, pre-experimental confirmation of probe performance [8].
The Researcher's Toolkit: Essential Reagents for Probe QC
Table 2: Research Reagent Solutions for Probe Quality Control
| Reagent/Material | Function in QC Process |
|---|---|
| Labeled Nucleotides | (e.g., Digoxigenin-UTP, Fluorescein-UTP) Incorporated during probe synthesis to generate the detectable signal. |
| In Vitro Transcription Kit | Provides optimized buffers and enzymes for efficient synthesis of RNA probes. |
| Spin Columns / Precipitation Kits | For purifying synthesized probes by removing unincorporated nucleotides and enzymes. |
| Spectrophotometer / Fluorometer | Instrumentation for quantifying nucleic acid and label concentration, and assessing purity. |
| Gel Electrophoresis System | For visualizing probe integrity and confirming successful synthesis and purification. |
| Nylon/Nitrocellulose Membrane | Solid support for performing dot-blot assays for functional validation of probe specificity. |
Troubleshooting Common Probe QC Issues
Even with standardized protocols, issues can arise. The diagram below outlines a logical workflow for diagnosing and addressing common probe quality problems.
Detailed Experimental Protocol: Probe Synthesis and QC
This protocol outlines the synthesis of labeled RNA probes via in vitro transcription and the subsequent quality control steps, adapted from established FISH methods [68].
Probe Synthesis via In Vitro Transcription
- Materials: Linearized DNA template (1 µg), 10x transcription buffer, RNase-free water, 10x labeled nucleotide mix (e.g., 10 mM ATP, CTP, GTP, 6.5 mM UTP, 3.5 mM Digoxigenin-UTP), 0.1M DTT, RNase inhibitor, appropriate RNA polymerase (T7, T3, or SP6).
- Procedure:
- Set Up Reaction: In a nuclease-free microcentrifuge tube, combine the following on ice: 1 µg linearized template, 2 µL 10x transcription buffer, 2 µL 10x labeled nucleotide mix, 1 µL RNase inhibitor, 1 µL 0.1M DTT, 1 µL RNA polymerase. Adjust volume to 20 µL with RNase-free water.
- Incubate: Mix gently and incubate at 37°C for 2 hours.
- DNase Treatment: Add 1 µL of DNase I (RNase-free) and incubate at 37°C for 15 minutes to digest the DNA template.
- Purify Probe: Purify the probe using a spin column or by ethanol precipitation to remove unincorporated nucleotides, enzymes, and salts. Elute or resuspend the probe in 50 µL of RNase-free TE buffer or hybridization buffer.
- Storage: Aliquot and store probes at -80°C to prevent degradation.
Quality Control Assessment
- Spectrophotometry:
- Dilute 2 µL of the purified probe in 98 µL of TE buffer (1:50 dilution).
- Measure absorbance at 230nm, 260nm, 280nm, and the maximum absorbance for the label (e.g., ~490 nm for Fluorescein).
- Calculate nucleic acid concentration: [Probe] (ng/µL) = A260 × Dilution Factor × Conversion Factor (40 for RNA; 50 for dsDNA). Assess purity via A260/A280 and A260/A230 ratios.
- Gel Electrophoresis:
- Prepare a 1-2% denaturing agarose gel (with formaldehyde for RNA probes).
- Mix 100-200 ng of probe with denaturing loading dye, heat at 65°C for 5-10 minutes, and load on the gel alongside an RNA ladder.
- Run the gel at 5-6 V/cm until adequate separation is achieved.
- Visualize under UV light. A single, sharp band of the expected size confirms probe integrity and purity.
Implementing a rigorous and standardized quality control pipeline for FISH probes is non-negotiable for generating scientifically sound and reproducible data in microbial detection research. By systematically evaluating probes for labeling efficiency, purity, concentration, and functional specificity, researchers can confidently proceed with their FISH experiments, knowing that the fundamental reagent is of the highest quality. This proactive approach to probe QC minimizes experimental variability, reduces costly reagent waste, and accelerates the path to reliable discovery in both academic and drug development settings.
Combating Photobleaching and Preserving Signal During Microscopy
Fluorescence in situ hybridization (FISH) has emerged as a cornerstone technique in molecular cytogenetics, enabling the precise localization of specific nucleic acid sequences within cellular and tissue contexts [51]. However, the full potential of FISH, particularly in advanced applications like microbial detection, is often compromised by photobleaching—the irreversible loss of fluorescence signal upon repeated light exposure. This phenomenon becomes particularly problematic in high-resolution imaging, time-lapse studies, and multiplexed analyses where signal integrity is paramount.
The signal attenuation caused by photobleaching is not merely an inconvenience but a significant technical barrier that can lead to false negatives, reduced detection efficiency, and compromised data quality [70]. In microbial detection research, where target organisms may be present in low abundance, preserving signal throughout the imaging process becomes critical for accurate identification and quantification. This application note outlines current methodologies and practical protocols to combat photobleaching and preserve signal integrity in FISH-based microbial detection research.
Technical Strategies for Signal Preservation
Automated Image Acquisition with Signal Enhancement
Recent advances in computational imaging have yielded sophisticated approaches that mitigate photobleaching by extracting maximum information from minimal photon collection. An automated workflow integrating dynamic signal enhancement addresses the dual challenges of signal attenuation and background noise [70]. This method employs an improved Cycle-Consistent Generative Adversarial Network (Cycle-GAN) architecture incorporating residual connections and layer-wise supervision to model and compensate for complex signal characteristics without requiring prolonged light exposure.
Key performance metrics demonstrate the efficacy of this approach, with reported increases in weak signal brightness by 49.02%, edge gradients by 48.61%, and contrast improvement index (CII) by 32.52%, while maintaining a structural similarity index (SSIS) of 0.996 relative to original signals [70]. The method effectively resolves issues like signal fragmentation and uneven distribution, producing clearer, more continuous fluorescence signals that enhance detection accuracy while reducing light dose.
Table 1: Quantitative Performance Metrics of Automated Signal Enhancement
| Performance Parameter | Improvement | Significance |
|---|---|---|
| Weak Signal Brightness | +49.02% | Enhanced detectability of faint targets |
| Edge Gradients | +48.61% | Improved boundary definition |
| Contrast Improvement Index (CII) | +32.52% | Better signal-to-background separation |
| Structural Similarity Index (SSIM) | 0.996 | High fidelity to original signal characteristics |
Optimized Buffer Composition and Reagent Stability
The chemical environment during imaging profoundly influences fluorophore longevity. Systematic optimization of buffer composition has been shown to significantly improve photostability in multiplexed FISH applications like MERFISH (Multiplexed Error-Robust FISH) [40]. Specific modifications to buffer formulations can enhance photophysical properties including fluorophore longevity and effective brightness, particularly important for experiments extending across multiple days.
Empirical testing has identified optimal buffer components that maintain reagent stability throughout extended imaging sessions [40] [71]. These formulations mitigate the "aging" effect of reagents that otherwise leads to progressive signal degradation. For microbial FISH applications, implementing these optimized buffers can increase the usable imaging window, allowing for more extensive z-stacking, time-lapse imaging, or multiplexed target detection without signal loss.
Isotropic Light-Sheet Microscopy
Light-sheet fluorescence microscopy (LSFM) represents a paradigm shift in fluorescence imaging by fundamentally reducing light exposure. This technique confines excitation to the immediate vicinity of the focal plane, dramatically reducing out-of-focus illumination and the associated photobleaching [72]. Recent advancements have produced an isotropic, aberration-corrected LSFM capable of maintaining 850 nm resolution across cleared samples up to 1 cm³ while compatible with refractive indices from 1.33 to 1.56 [72].
The system employs an innovative design combining an air objective, meniscus lens, and axially swept light-sheet to achieve diffraction-limited resolution while minimizing spherical aberrations. A key feature is the implementation of a voice coil actuator for high-speed light-sheet sweeping (up to 100 frames per second), which further reduces photon dose per image while maintaining field of view and resolution [72]. For microbial detection in complex samples, this approach offers unparalleled signal preservation throughout extended imaging sessions.
Figure 1: Isotropic Light-Sheet Microscopy Workflow
Practical Protocols for FISH Signal Preservation
Protocol: Automated Imaging with Real-Time Signal Enhancement
This protocol leverages computational approaches to maximize signal detection while minimizing light exposure, particularly beneficial for detecting low-abundance microbial targets.
Materials Required:
- Fluorescence microscope with motorized stage and z-axis control
- High-quality objectives appropriate for your FISH probes
- Computer with GPU acceleration capability
- Signal enhancement software (custom or commercial)
Procedure:
- Sample Positioning: Move the microscope to the target area without preliminary low-magnification scanning to reduce unnecessary exposure.
- Focus Optimization: Program the stage to descend in 0.8 µm increments, capturing approximately 150 images to determine optimal focus using an energy gradient algorithm.
- Image Acquisition: Capture 11 images at 0.44 µm intervals within a 2.2 µm z-stack range for each field of view.
- Signal Enhancement: Process images through the Cycle-GAN-based fluorescence signal compensation network incorporating:
- Residual connections for stable training
- Multi-layer convolution modules for feature extraction
- Hierarchical supervision for accurate signal modeling
- Field Repetition: Repeat the process for all sample areas to ensure complete coverage.
This automated process improves efficiency, reproducibility, and image quality while reducing overall photodamage [70].
Protocol: Buffer Optimization for Enhanced Photostability
This protocol outlines the preparation and use of optimized imaging buffers to prolong fluorophore longevity, particularly crucial for time-series imaging of microbial communities.
Materials Required:
- High-purity reagents for buffer preparation
- Reducing agents (e.g., trolox, ascorbic acid)
- Oxygen scavenging systems
- Mounting media compatible with your sample type
Procedure:
- Base Buffer Preparation: Prepare your standard imaging buffer appropriate for your sample type.
- Additive Optimization: Systematically test combinations of photostabilizing agents including:
- Oxygen scavenging systems (e.g., glucose oxidase-catalase)
- Triplet-state quenchers (e.g., trolox, cyclooctatetraene)
- Reducing agents (e.g., ascorbic acid, methyl viologen)
- Performance Validation: Compare signal longevity across buffer formulations using standardized bleaching assays.
- Sample Mounting: Apply optimized buffer to samples immediately before imaging.
- Sealing: Properly seal coverslips to prevent buffer evaporation and oxygen penetration during extended imaging.
These modifications can substantially improve photostability and effective brightness for commonly used FISH fluorophores [40].
Protocol: Sample Preparation to Minimize Background Fluorescence
Proper sample preparation is foundational to achieving high signal-to-noise ratios, reducing the need for excessive light exposure that accelerates photobleaching.
Materials Required:
- Freshly prepared fixative solutions (e.g., 4% formaldehyde or PFA in PBS)
- Permeabilization agents (e.g., Triton X-100, Tween-20)
- Hypotonic solutions (e.g., potassium chloride) for blood smears
- Appropriate protease inhibitors
Procedure:
- Fixation Optimization:
- For cells and tissues, use freshly prepared 4% formaldehyde or paraformaldehyde in PBS
- Avoid both under-fixation (incomplete cellular structure preservation) and over-fixation (excessive cross-linking)
- For blood smears, include hypotonic solutions during fixation to reduce background
- Permeabilization:
- Use detergents at 0.1% concentration (e.g., Tween-20 or Triton X-100) to enhance tissue permeability
- Titrate concentration and duration to balance probe access and structural preservation
- Pre-treatment:
- For FFPE tissues, use controlled enzymatic digestion (e.g., pepsin or proteinase K)
- Avoid both insufficient pre-treatment (leading to autofluorescence) and over-digestion (damaging target sequences)
- Wash Steps:
- Perform stringent post-hybridization washes with freshly prepared buffers
- Optimize stringency by adjusting pH, temperature, and incubation time
- Include ethanol washes to reduce autofluorescence in tissues or cells
Proper sample preparation significantly reduces background fluorescence, enabling lower exposure times and reducing photobleaching [1] [73].
Table 2: Research Reagent Solutions for FISH Signal Preservation
| Reagent Category | Specific Examples | Function in Signal Preservation |
|---|---|---|
| Photostabilizing Buffers | Oxygen scavenging systems, Triplet-state quenchers | Extend fluorophore longevity by reducing photodamage |
| Fixatives | Fresh 4% formaldehyde, Carnoy's solution | Preserve cellular structure while maintaining target accessibility |
| Permeabilization Agents | Triton X-100, Tween-20 (0.1%) | Enable probe penetration without excessive structural damage |
| Enzymatic Pre-treatment | Pepsin, Proteinase K | Unmask target sequences while preserving nucleic acid integrity |
| Stringent Wash Buffers | SSC solutions, Freshly prepared wash buffers | Remove non-specifically bound probes to reduce background |
| Mounting Media | Antifade reagents, Glycerol-based media | Reduce photobleaching during imaging |
Implementation Workflow and Technical Integration
Figure 2: Integrated Signal Preservation Workflow
Successful implementation of photobleaching mitigation requires a systematic approach integrating multiple strategies throughout the experimental pipeline. The workflow begins with optimized sample preparation to minimize initial background fluorescence, followed by careful attention to probe design and hybridization conditions [40] [73]. Selection of appropriate photostable imaging buffers creates a protective chemical environment during data acquisition [40]. The choice of imaging modality—whether widefield, confocal, or light-sheet microscopy—should be matched to experimental requirements and photobleaching sensitivity [72]. During acquisition, exposure parameters should be optimized to balance signal detection with preservation. Finally, computational enhancement can extract maximum information from the collected photons [70].
This integrated approach provides cumulative benefits, with each step contributing to reduced photodamage and enhanced signal preservation. For microbial detection applications, where absolute signal levels may be low, implementing this comprehensive workflow can dramatically improve detection efficiency, quantitative accuracy, and experimental reproducibility.
Photobleaching remains a significant challenge in FISH-based microbial detection, but current methodologies offer powerful approaches to mitigate its effects. Through integrated implementation of computational signal enhancement, optimized imaging buffers, advanced microscopy modalities, and rigorous sample preparation, researchers can significantly extend the usable imaging window and improve signal detection. The protocols outlined herein provide practical guidance for implementing these strategies in laboratory settings, enabling more robust and reliable FISH analyses for microbial detection and characterization.
Preventing Cross-Contamination for Reproducible Results
Cross-contamination represents a significant challenge in fluorescence in situ hybridization (FISH) experiments, potentially compromising data integrity and experimental reproducibility. In microbial detection research, where FISH serves as a powerful tool for identifying and characterizing microorganisms, preventing contamination is paramount for obtaining reliable results [29] [17]. This application note provides detailed methodologies and quantitative guidelines to minimize cross-contamination risks throughout the FISH workflow, ensuring reproducible and accurate outcomes for researchers, scientists, and drug development professionals.
The fundamental principle of FISH involves using fluorescently labeled DNA probes that bind to complementary target sequences, which are then visualized via fluorescence microscopy [29]. Even minor contamination at any stage—from sample preparation through final analysis—can generate false positives or obscure genuine signals. By implementing the rigorous protocols outlined in this document, laboratories can significantly enhance the reliability of their FISH-based microbial detection assays.
Critical Control Points in the FISH Workflow
The FISH procedure consists of multiple sequential steps where cross-contamination may occur. Understanding and controlling these critical points is essential for maintaining experimental integrity.
Sample Preparation and Handling
Initial sample handling establishes the foundation for contamination-free FISH. For microbial samples, proper collection and preservation are crucial:
- Cell Culture and Harvesting: For cell samples, culture, harvest, and fix to arrest cell division. FISH can also be performed on uncultured materials including blood, bone marrow, and amniocytes [29]. The harvested, fixed-cell pellet should be resuspended in Carnoy's solution or alternative fixatives [29].
- Tissue Sectioning: For tissue samples, section tissue blocks into thin ribbons, mount onto slides, and bake overnight. Treat with organic solvents to remove paraffin wax, followed by pre-treatment and digestion to optimize tissue structure for FISH [29].
- Slide Preparation: Prepare sample slides fresh on the same day as the FISH assay will be performed [29]. Use dedicated workspace and tools for pre-hybridization steps to prevent amplicon contamination.
Probe Handling and Hybridization
Probe-related contamination can lead to false positive signals:
- Probe Storage and Handling: Store FISH probes according to manufacturer specifications and keep them in the dark as much as possible during use, as they can degrade when exposed to light [29]. Use separate aliquots for each experiment to prevent repeated freeze-thaw cycles.
- Hybridization Conditions: Employ automated hybridization units such as ThermoBrite or hotplates for denaturation to ensure consistent temperature control [29]. Implement proper quality control measures to verify denaturation temperatures, as some units may have inaccurate digital readouts [29].
- Workspace Segregation: Physically separate pre-hybridization, hybridization, and post-hybridization areas. Use dedicated equipment and supplies for each phase of the procedure.
Post-Hybridization Washes and Visualization
Inadequate washing can leave unbound probe that contributes to background noise:
- Stringent Washes: Perform post-hybridization washes in 0.4xSSC solution at 72°C for two minutes, followed by a second wash in 2xSSC with 0.05% Tween-20 at room temperature for 30 seconds [29]. These concentrations may vary depending on the specific product—always consult the manufacturer's instructions for use (IFU) [29].
- Signal Verification: Include appropriate controls in each experiment to distinguish true hybridization from non-specific binding or contamination.
Quantitative Assessment of Contamination Impact
The table below summarizes key quantitative findings from literature regarding the impact of procedural variables on cell viability and potential contamination in FISH experiments:
Table 1: Quantitative Impact of FISH Procedures on Microbial Viability and Potential Contamination Risks
| Experimental Variable | Impact Measurement | Contamination Implication | Citation |
|---|---|---|---|
| Live-FISH Treatment | One-order of magnitude reduction in viable cells | Highlights sensitivity of microbial communities to procedural stress | [5] |
| Taxon-Specific Viability Retention | 501 ASVs retained viability post-treatment | Demonstrates differential survival requiring tailored contamination controls | [5] |
| Phylum-Specific Effects | Bacillota and Planctomycetota more resilient than Acidobacteriota (reduced by 5 orders of magnitude) | Indicates need for taxon-specific contamination monitoring | [5] |
| Hybridization Temperature | 37°C (±1°C) in humid environment | Standardized conditions reduce variability and cross-experiment contamination | [29] |
| Denaturation Temperature | 75°C ±1°C for 2-5 minutes | Precise temperature control ensures specific hybridization reducing false positives | [29] |
Detailed Experimental Protocol for Contamination-Free FISH
Pre-Hybridization Procedures
Slide Preparation:
- Prepare microscope slides fresh on the day of the FISH assay [29]
- For tissue samples: Bake overnight, deparaffinize with organic solvents, and perform pre-treatment digestion [29]
- For cell samples: Culture, harvest, and fix cells in Carnoy's solution [29]
- Use separate workspace and tools for different sample types
Probe Preparation:
- Thaw probe aliquots on ice and protect from light [29]
- Prepare probe mixtures in dedicated clean areas
- Use aerosol-resistant barrier tips for all liquid handling
Hybridization Procedure
Probe Application:
Denaturation and Hybridization:
Post-Hybridization Processing
Washing Steps:
Counterstaining and Visualization:
- Apply fluorescent DNA counterstain such as DAPI after washing completion [29]
- Analyze using fluorescence microscope with appropriate filters (DAPI, Texas Red, FITC, etc.) [29]
- Implement standardized counting protocols (e.g., two technologists counting 100 interphase cells each from different hybridization areas) [29]
Workflow Visualization
The following diagram illustrates the complete FISH procedure with integrated contamination control points:
Figure 1: FISH experimental workflow with critical contamination control points highlighted.
Research Reagent Solutions
Table 2: Essential Materials and Reagents for Contamination-Free FISH Experiments
| Reagent/Equipment | Function | Contamination Control Specification |
|---|---|---|
| Carnoy's Solution | Cell fixation and preservation | Fresh preparation for each experiment; proper storage conditions |
| FISH Probes | Target sequence detection | Aliquot storage; protection from light; avoid repeated freeze-thaw cycles |
| Hybridization Buffer (0.9M NaCl, 20mM Tris-HCl, SDS, formamide) | Enables specific probe binding | Prepare fresh from sterile stock solutions; filter sterilize if required |
| SSC Wash Solutions (0.4xSSC and 2xSSC) | Remove unbound probe | Fresh preparation for each experiment; pH adjusted to 7.0 |
| DAPI Counterstain | Nuclear/chromosomal staining | Filter before use; aliquot to prevent microbial growth |
| ThermoBrite/Hotplate | Temperature control for denaturation/hybridization | Regular calibration verification; dedicated equipment for FISH |
| Fluorescence Microscope | Signal visualization | Regular maintenance; decontamination between samples |
Implementing rigorous contamination control measures throughout the FISH workflow is essential for obtaining reproducible results in microbial detection research. By adhering to the detailed protocols, quantitative guidelines, and reagent specifications outlined in this application note, researchers can significantly enhance the reliability of their FISH-based assays. The integration of these practices within a broader research framework ensures that FISH remains a powerful and trustworthy technique for investigating microbial communities in various contexts, from clinical diagnostics to environmental microbiology.
Ensuring Accuracy: FISH Validation, Comparative Analysis, and Position in the Diagnostic Landscape
A Framework for Preclinical FISH Assay Validation
Fluorescence in situ hybridization (FISH) is a powerful tool for the detection of specific DNA and RNA sequences within individual cells, finding applications in karyotyping, cancer diagnosis, species specification, and gene expression analysis [33]. Within microbial detection research, its ability to provide high-resolution visualization of pathogens makes it invaluable. However, before employing any FISH assay in clinical or research practice, a rigorous preclinical validation is mandatory to ensure its reliability, accuracy, and reproducibility [74]. The validation process confirms the analytical sensitivity, specificity, and precision of the assay, establishing normal cutoffs and abnormal reference ranges. This document outlines a comprehensive, broadly applicable framework for the preclinical validation of FISH assays, with specific considerations for microbial detection.
Core Validation Experiments
A robust preclinical validation should consist of four consecutive experiments designed to systematically evaluate all critical assay parameters [74]. The table below summarizes the objectives and key outputs of each experiment.
Table 1: Core Experiments for Preclinical FISH Assay Validation
| Experiment | Primary Objective | Specimens Used | Key Outputs |
|---|---|---|---|
| 1. Familiarization | Assess initial probe performance and measure basic analytic sensitivity and specificity. | Normal metaphase cells (e.g., from blood). | Initial performance data; probe behavior confirmation. |
| 2. Pilot Study | Establish a preliminary normal cutoff and analytic sensitivity on the intended tissue type. | A variety of normal and abnormal specimens of the target tissue. | Preliminary normal cutoff; initial standard operating procedure (SOP). |
| 3. Clinical Evaluation | Finalize validation parameters by simulating clinical practice conditions. | A larger series of normal and abnormal specimens. | Final normal cutoff; abnormal reference range; finalized SOP. |
| 4. Precision | Measure the inter-assay reproducibility and reliability. | Normal and abnormal specimens tested over multiple days. | Inter-day coefficient of variation; confirmation of reportable ranges. |
The following workflow diagram illustrates the sequential nature of this validation framework and the key activities at each stage.
Experimental Protocols and Methodologies
Detailed FISH Procedure
The following protocol is adapted for microbial detection and covers the essential steps from sample preparation to imaging [75].
Sample Preparation (Slide Pretreatment)
- Fixation: Begin with an appropriate cell fixation step.
- RNase Treatment: To reduce background, incubate slides with 100 µg/mL RNase A in 2x SSC buffer at 37°C for 1 hour. Wash twice in 2x SSC for 5 minutes each [75].
- Permeabilization: Rinse slides in 10 mM HCl, then incubate with 40 U/mL pepsin in 10 mM HCl at 37°C for 10 minutes. Rinse in deionized water [75].
- Post-fixation: Fix slides in 4% paraformaldehyde for 10 minutes, followed by two 5-minute washes in 2x SSC [75].
- Dehydration: Dehydrate slides through an ethanol series (70%, 80%, 95%) for 2 minutes each and air dry [75].
Hybridization
- Probe Preparation: Prepare a hybridization mixture containing 50% formamide, 10% dextran sulfate, 0.1% SDS, the labeled probe (0.5-1.5 ng/µL), and 300 ng/µL of sheared salmon sperm DNA in 2x SSC. Heat the mixture to 70°C for 10 minutes and then immediately place on ice [75].
- Denaturation: Apply 30 µL of the hybridization mixture to the slide, cover with a plastic coverslip, and denature the specimen and probe together on a hot module at 65-70°C for 5 minutes [75].
- Hybridization: Gradually lower the temperature to 37°C and incubate in a humidified chamber overnight (approximately 16 hours) [75].
Post-Hybridization Washing and Detection
- Stringency Washes: Remove the coverslip by washing in 2x SSC. Then, wash slides twice in a buffer containing 20% formamide and 0.1x SSC at 40°C for 5 minutes each. Follow with a wash in 0.1x SSC at 40°C for 5-15 minutes, and another in 2x SSC at 40°C for 5-15 minutes. Cool slides to room temperature [75].
- Detection: For probes labeled with haptens (e.g., biotin), equilibrate slides in detection buffer. Block nonspecific sites with 5% bovine serum albumin (BSA) in detection buffer for 20-30 minutes. Incubate with the detection compound (e.g., 5 µg/mL streptavidin-Cy3 in blocking buffer) for 30-60 minutes. Wash three times in 2x SSC for 5 minutes each [75].
- Counterstaining and Mounting: Counterstain with 2 µg/mL DAPI for 10 minutes, perform a brief rinse, and mount in an anti-fade medium [75].
- Imaging: Analyze slides using a fluorescence microscope equipped with appropriate filter sets [75].
Simplified ECHO-FISH Protocol
For a rapid turnaround, the ECHO-FISH method using exciton-controlled hybridization-sensitive fluorescent oligodeoxynucleotide (ECHO) probes can be employed. This protocol is significantly simplified, requiring only 25 minutes from fixation to mounting and omitting stringency washing steps due to the low fluorescent emission of unhybridized probes [33]. The protocol involves fixation, application of the ECHO probe, hybridization, and mounting, enabling rapid detection of specific microbial RNA or DNA sequences.
Key Performance Metrics and Data Analysis
Throughout the validation experiments, quantitative data must be collected to define the assay's performance. The following table outlines essential metrics and their calculation methods.
Table 2: Key Performance Metrics for FISH Assay Validation
| Metric | Description | Calculation / Acceptable Range |
|---|---|---|
| Analytic Sensitivity | The ability of the assay to correctly identify true positives. | Percentage of known positive samples that test positive. |
| Analytic Specificity | The ability of the assay to correctly identify true negatives. | Percentage of known negative samples that test negative. |
| Normal Cutoff | The threshold above which a signal is considered positive. | Mean + 3 standard deviations of signals from normal specimens. |
| Recovery Rate | Efficiency of the detection process. | 52-101% (as demonstrated in relevant validations) [76]. |
| Precision (Inter-day CV) | Measure of reproducibility over time. | Coefficient of variation ≤16% is achievable [76]. |
The Scientist's Toolkit
A successful FISH assay relies on specific reagents and equipment. The table below lists essential solutions and their functions.
Table 3: Research Reagent Solutions for FISH Assays
| Reagent / Solution | Function | Example |
|---|---|---|
| 20X SSC Buffer | Provides the correct ionic strength and pH for hybridization and washing. | 3 M NaCl, 0.3 M sodium citrate, pH 7.0 [75]. |
| Formamide | A denaturing agent used in hybridization mix and stringent washes to control specificity. | Used at 50% in hybridization mix and 20% in post-hybridization washes [75]. |
| Blocking Agent (BSA) | Reduces non-specific binding of detection reagents to the sample. | 5% BSA in detection buffer [75]. |
| Detection Compounds | Molecules that bind to the labeled probe and generate a fluorescent signal. | Streptavidin conjugated to fluorophores like Cy3, Alexa Fluor dyes [75] [32]. |
| DAPI | A fluorescent counterstain that binds to DNA, labeling all cell nuclei. | Used at 2 µg/mL to visualize overall cellular architecture [75]. |
| Signal Amplification Kits | Enhance fluorescence signal for low-abundance targets, improving sensitivity. | SuperBoost Kits offering 10-200x sensitivity of standard methods [32]. |
The implementation of this four-experiment framework—comprising Familiarization, Pilot Study, Clinical Evaluation, and Precision testing—provides a structured path for the thorough preclinical validation of FISH assays [74]. By adhering to detailed protocols for sample processing, hybridization, and detection, and by rigorously quantifying performance metrics such as sensitivity, specificity, and reproducibility, researchers can ensure the generation of reliable and meaningful data. This disciplined approach is fundamental for advancing the use of FISH in critical areas like microbial detection research, drug development, and clinical diagnostics.
Establishing Analytic Sensitivity, Specificity, and Normal Cut-Offs
Fluorescence in situ hybridization (FISH) is a powerful cytogenetic technique that enables the detection, identification, and localization of specific nucleic acid sequences within intact cells using fluorescently labeled probes [77]. For clinical and research applications, establishing robust performance characteristics—including analytic sensitivity, specificity, and normal cut-off values—is paramount for ensuring accurate and reliable results. This protocol outlines standardized procedures for determining these critical validation parameters within the context of microbial detection, providing researchers and laboratory professionals with a framework for implementing FISH assays that meet rigorous quality standards.
The fundamental principle of FISH involves hybridizing fluorescently labeled nucleic acid probes to complementary target sequences, allowing visualization through fluorescence microscopy or quantification via flow cytometry [78]. As the technology continues to evolve with automation and novel probe designs, the demand for standardized validation protocols has grown, particularly for applications in clinical diagnostics, pharmaceutical development, and microbial ecology research [27] [45].
Key Definitions and Principles
Performance Metrics
- Analytic Sensitivity: The probability that the FISH assay will correctly identify a true positive result, calculated as the proportion of positive samples that test positive. High sensitivity is crucial for detecting low-abundance targets [79] [78].
- Analytic Specificity: The probability that the FISH assay will correctly identify a true negative result, calculated as the proportion of negative samples that test negative. This parameter reflects the assay's ability to avoid cross-reactivity with non-target sequences [79] [78].
- Normal Cut-Off Value: The statistically determined threshold that distinguishes positive from negative results, representing the minimum number of positive signals required to define a confident FISH diagnosis. This value depends on technical parameters, nuclear size, probe strategy, and potential sources of spurious signals [78].
Technical Considerations Influencing Performance
Several technical factors significantly impact the sensitivity and specificity of FISH assays:
- Probe Design: Probe length, base composition, and fluorescent labeling affect hybridization efficiency and specificity [45] [77].
- Sample Quality: Fixation method, sample age, and storage conditions influence nucleic acid preservation and accessibility [78].
- Hybridization Stringency: Temperature, salt concentration, and formamide percentage control the balance between specific and non-specific hybridization [80] [77].
- Signal Detection: Microscope resolution, fluorescence intensity, and background autofluorescence affect result interpretation [78] [81].
Experimental Protocols for Parameter Establishment
Determining Analytic Sensitivity and Specificity
Purpose: To establish the probability of true positive detection (sensitivity) and true negative detection (specificity) for the FISH assay.
Materials:
- Well-characterized reference samples with known positive and negative status
- Appropriate FISH probes and hybridization reagents
- Fluorescence microscope with appropriate filter sets
- Data recording forms or digital capture system
Procedure:
- Sample Selection: Obtain a minimum of 50 positive and 50 negative samples confirmed by an established reference method. Ensure samples represent the expected range of target abundance and sample types [79].
- Blinded Testing: Code all samples to ensure blinded analysis. Process all samples identically using the standardized FISH protocol.
- Hybridization and Detection: Perform FISH according to established protocols, ensuring consistent hybridization conditions, washing stringency, and detection parameters across all samples [80] [45].
- Independent Interpretation: Have at least two qualified technologists evaluate each sample independently, recording the presence or absence of positive signals according to predefined criteria.
- Data Analysis: Compare FISH results with reference method results using a 2×2 contingency table.
- Calculation:
- Sensitivity = [True Positives / (True Positives + False Negatives)] × 100
- Specificity = [True Negatives / (True Negatives + False Positives)] × 100
Example from Literature: In malaria detection, FISH demonstrated 85.6% sensitivity and 96.2% specificity compared to PCR as reference [81].
Establishing Normal Cut-Off Values
Purpose: To determine the threshold that distinguishes positive from negative results, minimizing false positives and false negatives.
Materials:
- Normal control samples (minimum of 20-50 individuals)
- Test samples with known abnormalities
- Standardized FISH protocol materials
- Statistical software for data analysis
Procedure:
- Control Sample Analysis: Process normal control samples using the standardized FISH protocol. For each sample, count the number of cells showing abnormal signals out of a predefined total (typically 200-1000 cells) [78].
- Data Collection: Record the percentage of cells with abnormal signals for each normal control.
- Statistical Analysis: Calculate the mean and standard deviation of the false-positive rate in normal controls.
- Cut-Off Determination: Establish the cut-off value as the mean percentage of abnormal cells in controls plus three standard deviations. This should encompass 99.7% of normal samples if normally distributed [78].
- Validation: Apply the established cut-off to test samples with known abnormalities to confirm appropriate segregation of positive and negative cases.
- Documentation: Clearly document the validated cut-off value, sample size used for determination, and statistical parameters in the laboratory procedure manual.
Critical Considerations:
- The cut-off value depends on technical parameters, nuclear size, probe strategy, and potential sources of spurious signals [78].
- For fusion detection probes, normal cut-offs are typically set at 1-5%, while for deletion probes, cut-offs may range from 5-20% depending on probe design and target [79].
- For quantitative applications, establish a standard curve using samples with known target concentrations [82].
Protocol for Flow-FISH Validation
Purpose: To establish sensitivity, specificity, and cut-offs for FISH combined with flow cytometry, enabling high-throughput microbial quantification.
Materials:
- Bacterial strains (target and non-target species)
- Species-specific FISH probes
- Flow cytometer with appropriate laser and detector configuration
- Fixation reagents (paraformaldehyde, ethanol)
- Hybridization buffer and wash buffer
Procedure:
- Sample Preparation: Fix cells in 4% paraformaldehyde for gram-negative or ethanol for gram-positive bacteria [80] [82].
- Probe Hybridization: Apply fluorescently labeled oligonucleotide probes targeting 16S or 23S rRNA. Optimize hybridization temperature and time based on probe characteristics [45] [82].
- Flow Cytometry Analysis: Analyze samples using flow cytometry, establishing gating parameters based on positive and negative controls.
- Signal Threshold Establishment: Determine positive signal thresholds using unstained cells and cells hybridized with nonsense probes.
- Quantification: Analyze a minimum of 10,000 events per sample for statistically significant quantification [82].
- Data Interpretation: Use established cut-offs to differentiate between positive and negative populations.
Validation Notes: The optimized Flow-FISH protocol can reduce hybridization times to 1.5 hours and eliminate centrifugation steps that might lead to cell loss [82].
Data Presentation and Analysis
Performance Characteristics of FISH Assays
Table 1: Established Performance Characteristics of FISH Across Applications
| Application | Sensitivity (%) | Specificity (%) | Cut-Off Criteria | Reference Method |
|---|---|---|---|---|
| Malaria detection [81] | 85.6 | 96.2 | Microscopic confirmation | PCR |
| HER2 testing in breast cancer [27] | 95.0 | 97.0 | Automated scoring | Manual FISH |
| HER2 testing in gastric cancer [27] | 100 | 100 | Automated scoring | Manual FISH |
| Bacterial identification with PNA probes [45] | 96-99.9 | >99 | FRET-based signal | Culture and sequencing |
Statistical Analysis Framework
Table 2: Statistical Parameters for FISH Validation
| Parameter | Calculation Formula | Acceptance Criteria | Example Values |
|---|---|---|---|
| Analytic Sensitivity | TP / (TP + FN) × 100 | ≥95% for diagnostic applications | 85.6-100% [27] [81] |
| Analytic Specificity | TN / (TN + FP) × 100 | ≥95% for diagnostic applications | 96.2-100% [27] [81] |
| Positive Predictive Value | TP / (TP + FP) × 100 | Varies by disease prevalence | 90.6% for malaria FISH [81] |
| Negative Predictive Value | TN / (TN + FN) × 100 | Varies by disease prevalence | 94.0% for malaria FISH [81] |
| Diagnostic Accuracy | (TP + TN) / Total × 100 | ≥90% | 93.0% for malaria FISH [81] |
Visualization of Experimental Workflows
FISH Validation Workflow
FISH Validation Workflow: This diagram illustrates the sequential process for establishing analytic sensitivity, specificity, and normal cut-offs for FISH assays.
Flow-FISH Integration Protocol
Flow-FISH Integration: This workflow details the integration of FISH with flow cytometry for high-throughput microbial quantification.
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for FISH Validation
| Reagent/Category | Function/Purpose | Examples/Specifications |
|---|---|---|
| Probe Types | Target-specific nucleic acid detection | DNA probes, PNA probes (higher affinity, mismatch sensitivity) [45] |
| Fixation Reagents | Cell structure preservation and permeabilization | Paraformaldehyde (gram-negative), ethanol (gram-positive) [80] [82] |
| Hybridization Buffer | Controlled stringency environment for specific hybridization | Varying salt concentrations, formamide percentage [80] [77] |
| Signal Amplification Systems | Enhanced detection sensitivity | CARD-FISH, FRET-based probes [45] [77] |
| Counterstains | Nuclear and cellular structure visualization | DAPI, propidium iodide [78] [82] |
| Mounting Media | Signal preservation and fluorescence stability | Antifade mounting media [78] |
| Automation Platforms | Standardization and throughput enhancement | Leica BOND-III [27] |
Troubleshooting and Quality Assurance
Common Technical Challenges
- High Background Fluorescence: Optimize washing stringency, reduce probe concentration, and include formamide in hybridization buffer [78] [77].
- Weak or Absent Signals: Increase probe concentration, reduce washing stringency, verify probe penetration, and check fluorophore integrity [78].
- Inconsistent Results Between Operators: Implement standardized counting procedures, provide comprehensive training, and utilize automated scanning systems [27] [78].
- False-Positive Signals in Normal Controls: Re-evaluate cut-off values, increase sample size for normal baseline establishment, and include additional negative controls [78].
Quality Control Measures
- Probe Validation: Validate all new probe sets against known positive and negative controls before clinical or research use [79].
- Regular Proficiency Testing: Participate in external quality assurance programs and perform internal blinded sample testing [79] [78].
- Equipment Calibration: Regularly calibrate fluorescence microscopes and flow cytometers using standardized reference materials [78] [82].
- Documentation and Traceability: Maintain complete records of all validation procedures, reagent lots, and equipment maintenance [79].
Establishing rigorous analytic sensitivity, specificity, and normal cut-off values is fundamental to implementing reliable FISH assays in both research and clinical settings. The protocols outlined herein provide a standardized framework for validating FISH performance characteristics across diverse applications, from microbial detection to cancer diagnostics. By adhering to these guidelines and implementing robust quality control measures, researchers and laboratory professionals can ensure the generation of accurate, reproducible results that advance scientific understanding and support critical diagnostic decisions.
As FISH technology continues to evolve with innovations in probe design, signal amplification, and automation, the principles of rigorous validation remain constant. The integration of these established protocols with emerging methodologies will further enhance the utility of FISH as a powerful tool for nucleic acid visualization and quantification in complex biological systems.
Assessing Precision and Reproducibility Over Time
Within microbial detection research, the precision and reproducibility of Fluorescence In Situ Hybridization (FISH) are paramount for generating reliable, time-resistant data. FISH is a powerful molecular technique that uses fluorescently labeled nucleic acid probes to detect and localize specific nucleotide sequences within intact cells and tissues, allowing for the visualization of genetic material in its native spatial context [83]. This application note details protocols and data quantifying the performance of an optimized Flow-FISH method for enumerating probiotic bacteria, providing a framework for assessing technique stability in longitudinal studies. Ensuring that FISH results are both precise over repeated measurements and reproducible over time is a cornerstone for its successful application in clinical diagnostics, drug development, and microbial ecology.
Quantitative Assessment of Method Performance
The precision and reproducibility of the optimized Flow-FISH protocol were assessed by comparing its quantitative results against two established enumeration methods: plate count and Live/Dead (L/D) staining. The following tables summarize the key performance data.
Table 1: Comparison of Enumeration Results for Probiotic Strains [84]
| Strain | Plate Count (CFU) | L/D Staining (Cell Count) | Flow-FISH (Cell Count) | Notes |
|---|---|---|---|---|
| Lacticaseibacillus rhamnosus | Lower | Comparable | Higher | Flow-FISH counts higher than plate count, challenging the gold standard. |
| Lactiplantibacillus plantarum | Lower | Comparable | Higher | Flow-FISH counts higher than plate count, challenging the gold standard. |
| Bifidobacterium animalis subsp. lactis | Lower | Comparable | Higher | Flow-FISH counts higher than plate count, challenging the gold standard. |
| Commercial Product Blend | Lower | Comparable | Higher | Demonstrates method applicability in complex, multi-species samples. |
Table 2: Protocol Efficiency and Reproducibility Metrics [84]
| Parameter | Traditional FISH Protocols | Optimized Flow-FISH Protocol | Impact on Precision & Reproducibility |
|---|---|---|---|
| Hybridization Time | >10 hours | 1.5 hours | Reduces protocol drift and experimental variability over time. |
| Centrifugation Steps | Multiple | Eliminated | Minimizes cell loss, improving quantitative accuracy and replicate consistency. |
| Enumeration Method | Manual Microscopy | Automated Flow Cytometry | Standardizes cell counting, removing operator subjectivity and enhancing inter-lab reproducibility. |
| Viable Cell Count | Underestimated (Culturable only) | Higher (Includes VBNC states) | Provides a more accurate and consistent count of true viable cells. |
Experimental Protocol for Optimized Flow-FISH
This section provides a detailed methodology for the optimized Flow-FISH protocol used to generate the performance data, highlighting steps critical for ensuring precision and long-term reproducibility.
Sample Preparation and Fixation
- Rehydration: Weigh 100 mg of lyophilized probiotic sample and dilute at a 1:20 (w/v) ratio in a 0.1% peptone salt solution. Shake the suspension at 100 rpm for 60 minutes at room temperature [84].
- Fixation: Fix cells to stabilize and permeabilize them for probe entry. Specific fixative agents (e.g., paraformaldehyde) and concentrations should be optimized for the target gram-positive bacterial strains and consistently applied across experimental runs to ensure reproducible cell morphology and probe accessibility [77].
Probe Hybridization
- Probe Design: Use oligonucleotide probes fluorescently labeled and targeting specific sites of the ribosomal RNA (rRNA) of the microorganisms of interest. Probe sequence, length, and labeling are critical for specificity and must be rigorously controlled [84] [77].
- Hybridization: Apply the probe set to the fixed samples and incubate for 1.5 hours at the appropriate hybridization temperature. This significantly reduced time, compared to traditional protocols, enhances workflow consistency and minimizes potential for experimental error [84].
- Stringency Washes: Perform post-hybridization washes to remove unbound probe. The temperature and salt concentration of the wash buffer must be kept constant to ensure reproducible signal-to-noise ratios across different experimental days [77].
Analysis via Flow Cytometry
- Instrument Calibration: Prior to each session, calibrate the flow cytometer using standardized fluorescent beads. This is non-negotiable for ensuring day-to-day and instrument-to-instrument reproducibility of quantitative results [84].
- Data Acquisition: Analyze the stained samples using flow cytometry. The automated nature of this step provides high-throughput, objective quantification, eliminating the subjectivity associated with manual microscopic enumeration [84].
- Gating Strategy: Apply a consistent, predefined gating strategy to identify the target fluorescent population. Document and adhere to this strategy for all replicates and repeat experiments to maintain analytical precision [84].
Workflow and Logical Pathway
The following diagram illustrates the integrated experimental workflow of the optimized Flow-FISH protocol and the logical decision process for assessing its precision and reproducibility against established methods.
The Scientist's Toolkit: Research Reagent Solutions
A consistent set of high-quality reagents is fundamental to the precision of any FISH protocol. The table below lists essential materials and their functions, with an emphasis on items that bolster reproducibility.
Table 3: Essential Research Reagents for Flow-FISH [84] [77] [83]
| Reagent / Material | Function / Role in Assay | Critical for Precision/Reproducibility |
|---|---|---|
| Species-Specific FISH Probes | Fluorescently labeled oligonucleotides targeting rRNA for specific microbial identification. | Probe specificity and batch-to-batch consistency are the primary determinants of assay accuracy and cross-experiment reliability. |
| Peptide Nucleic Acid (PNA) Probes | Synthetic nucleic acid mimics with a peptide backbone offering higher affinity and specificity [83]. | Enhanced binding and resistance to enzymatic degradation reduce variability in signal intensity and background. |
| Fixation Agent (e.g., PFA) | Stabilizes cellular structures and permeabilizes cell walls for probe entry. | Concentration and fixation time must be standardized to ensure consistent cell permeability and morphology. |
| Hybridization Buffer | Provides the ionic strength and pH environment for specific probe binding. | Stringent control over buffer composition and hybridization temperature is key to reproducible signal-to-noise ratios. |
| Stringency Wash Buffer | Removes nonspecifically bound probe after hybridization. | Consistent salt concentration and temperature prevent unintended probe detachment and maintain low background. |
| Flow Cytometry Calibration Beads | Standardized particles for instrument performance validation. | Essential for normalizing fluorescence detection across different instruments and over time, ensuring quantitative reproducibility. |
The optimized Flow-FISH protocol, characterized by a shortened hybridization time and the elimination of centrifugation steps, demonstrates enhanced precision and reproducibility for the enumeration of viable microorganisms. By providing higher and potentially more accurate counts than the traditional plate count method and comparable results to L/D staining, this method establishes a robust framework for reliable longitudinal study in microbial research. Adherence to the detailed protocol, coupled with the use of standardized reagents and calibrated instrumentation, is critical for maintaining this high level of performance over time, making it a valuable tool for researchers and drug development professionals requiring consistent and trustworthy microbial detection data.
Fluorescence in situ hybridization (FISH) and Polymerase Chain Reaction (PCR) represent two foundational approaches in molecular diagnostics and microbial detection research. While both techniques are powerful tools for identifying pathogens and genetic abnormalities, they operate on fundamentally different principles, leading to distinct strengths and limitations. FISH utilizes fluorescently labeled nucleic acid probes to hybridize with complementary target sequences within cells, allowing for the visual localization and quantification of microorganisms within their morphological context [11]. In contrast, PCR employs a series of temperature cycles to enzymatically amplify specific DNA or RNA sequences, enabling the detection of minuscule amounts of genetic material with exceptional sensitivity [85]. This application note provides a systematic comparison of FISH and PCR methodologies, focusing on their comparative sensitivity, target range, and practical considerations for implementation in research and diagnostic settings. By synthesizing data from recent studies across clinical microbiology, oncology, and food safety, we aim to provide researchers and drug development professionals with evidence-based guidance for selecting the appropriate molecular technique for their specific application.
Performance Comparison: Quantitative Data Analysis
The selection between FISH and PCR requires careful consideration of their performance characteristics across different applications. The following tables summarize key comparative data from recent studies.
Table 1: Comparative Detection Sensitivity Across Applications
| Application Context | FISH Detection Rate | PCR Detection Rate | Reference Technique | Key Findings |
|---|---|---|---|---|
| Pediatric Sepsis [86] | 39.1% (36/92 samples) | 71.7% (66/92 samples) | Blood Culture (18% positive) | PCR demonstrated significantly higher detection rate; Both methods unaffected by antibiotic pretreatment |
| Mantle Cell Lymphoma [87] | 97% (34/35 cases) | 37% (13/35 cases) | Immunohistochemistry, Cytogenetics | FISH superior for detecting t(11;14) translocation due to scattered breakpoints |
| Canned Tuna Speciation [88] | N/A | 100% (Real-time PCR) | Morphology, DNA Barcoding | Real-time PCR outperformed multiplex PCR (29%) and mini-barcoding (33%) |
| Acute Leukemias [89] | N/A | RT-qPCR > Nested-PCR | Cytogenetics, Immunophenotyping | RT-qPCR showed higher sensitivity for fusion gene detection than Nested-PCR |
Table 2: Technical and Practical Characteristics Comparison
| Characteristic | FISH | PCR (Conventional & qPCR) |
|---|---|---|
| Target | Specific DNA/RNA sequences within intact cells | Specific DNA sequences (extracted nucleic acids) |
| Sensitivity | Lower (requires ~10â´ copies/cell) [11] | Very high (can detect single copies) [85] |
| Turnaround Time | 4-5 hours [86] | 3-6 hours (qPCR) [88] |
| Throughput Capacity | Lower (microscopy limits throughput) | High (especially qPCR platforms) |
| Spatial Information | Preserves cellular morphology and distribution [11] | No spatial context (homogenized sample) |
| Quantification | Semi-quantitative (based on cell counting) | Highly quantitative (qPCR, ddPCR) [85] |
| Multiplexing Capacity | Moderate (CLASI-FISH, DOPE-FISH) [11] | High (multiple primer/probe sets) |
| Amenable Sample Types | Tissue sections, blood smears, biofilms [11] | Extracted DNA/RNA from diverse samples |
Experimental Protocols
Standard FISH Protocol for Microbial Detection
The following protocol outlines the standard procedure for FISH-based detection of microorganisms in blood samples, adapted from the methodology used in pediatric sepsis studies [86].
Sample Preparation:
- Collect 200 µL of blood in EDTA tubes to prevent coagulation.
- Lyse red blood cells using 0.17 M ammonium chloride solution: Add 1 mL of ammonium chloride to the blood sample, mix gently, and incubate at room temperature for 10 minutes.
- Centrifuge at 5000 × g for 5 minutes and discard supernatant.
- Repeat the lysis step if the pellet remains red.
- Resuspend the resulting pale pink pellet in 20 µL of sterile deionized water.
- Transfer 10 µL of the suspension onto positively charged glass slides and air dry.
- Fix cells by applying 4% paraformaldehyde for 10 minutes at room temperature, then wash with PBS.
Hybridization:
- Prepare hybridization buffer containing appropriate salts, formamide, and detergents.
- Apply species-specific fluorescently labeled probes (e.g., CY3-labeled STA probe for Staphylococcus spp., CY3-labeled ENT 183 for Enterobacteriaceae, FITC-labeled EUB338 for all bacteria) [86].
- Add 10 µL of probe solution (50 ng/µL) to each sample area and cover with a coverslip.
- Incubate in a humidified chamber at appropriate hybridization temperature (varies by probe, typically 46°C) for 90 minutes to 3 hours.
Washing and Detection:
- Remove coverslips carefully and wash slides with pre-warmed washing buffer at 48°C for 10-15 minutes to remove unbound probe.
- Rinse briefly with distilled water and air dry in darkness.
- Apply antifading mounting medium containing DAPI (4',6-diamidino-2-phenylindole) for counterstaining of all nucleic acids.
- Visualize using epifluorescence microscopy with appropriate filter sets for each fluorophore.
- Interpret results: Positive signals show bright fluorescence at the cellular location of the target sequence.
PCR Protocol for Pathogen Detection
This protocol describes a nested multiplex real-time PCR approach for detecting bacterial pathogens in blood samples, as validated in sepsis diagnostics [86].
DNA Extraction:
- Collect 1.5 mL of blood in EDTA tubes and mix thoroughly.
- Extract microbial DNA using commercial blood DNA extraction kits (e.g., Blood Mini kit, A&A Biotechnology).
- Follow manufacturer's instructions, which typically include:
- Enzymatic lysis of human and microbial cells
- Protein degradation
- DNA binding to silica membrane
- Washing steps to remove inhibitors
- Elution in low-salt buffer or water
- Quantify DNA concentration using spectrophotometry (NanoDrop) and standardize to working concentration.
PCR Amplification: First Round (Multiplex PCR):
- Prepare reaction mixture containing:
- 5-50 ng template DNA
- 1X PCR buffer
- 2.5 mM MgClâ‚‚
- 200 µM dNTPs
- 0.5 µM of each primary primer
- 1.25 U DNA polymerase
- Nuclease-free water to 25 µL
- Perform thermal cycling:
- Initial denaturation: 95°C for 5 minutes
- 30 cycles of: 95°C for 30 seconds, 55-60°C for 30 seconds, 72°C for 45 seconds
- Final extension: 72°C for 7 minutes
Second Round (Real-time PCR):
- Prepare reaction mixture containing:
- 1-2 µL of first-round product
- 1X TaqMan Universal Master Mix
- Species-specific primers and probes (e.g., for Staphylococcus spp., Enterobacteriaceae)
- Nuclease-free water to 20 µL
- Perform real-time PCR:
- Initial denaturation: 95°C for 10 minutes
- 40 cycles of: 95°C for 15 seconds, 60°C for 1 minute (with fluorescence acquisition)
- Include appropriate controls: positive template controls, negative controls (no template), and inhibition control (human β-actin gene).
Data Analysis:
- Analyze amplification curves and determine cycle threshold (Ct) values.
- Identify species based on specific probe detection channels.
- Quantify bacterial load using standard curves if absolute quantification is required.
Research Reagent Solutions
Table 3: Essential Research Reagents for FISH and PCR Applications
| Reagent Category | Specific Examples | Function & Application Notes |
|---|---|---|
| FISH Probes | EUB338 (FITC-labeled) [86]STA probe (CY3-labeled) [86]ENT 183 (CY3-labeled) [86] | Target 16S rRNA for bacterial detection; Universal and species-specific identification; Fluorophore choice depends on microscope filter sets |
| Nucleic Acid Mimics (NAMs) | Peptide Nucleic Acids (PNA) [14]Locked Nucleic Acids (LNA) [14] | Enhanced hybridization affinity and specificity; Resistance to nuclease degradation; Improved cellular penetration |
| PCR Primers/Probes | Species-specific TaqMan probes [86]β-actin gene primers [86] | Enable real-time detection and quantification; Human gene targets serve as internal controls for inhibition detection |
| Nucleic Acid Extraction Kits | Blood Mini kit (A&A Biotechnology) [86]DNeasy Blood and Tissue Kit (Qiagen) [88] | Efficient isolation of microbial DNA from complex samples; Critical for PCR sensitivity and reproducibility |
| PCR Master Mixes | TaqMan Universal Master Mix [86]Platinum SuperFi II PCR Master Mix [89] | Provide optimized buffer conditions, enzymes, and dNTPs; Ensure high-fidelity amplification |
| Hybridization Buffers | Formamide-based hybridization solutions [11] | Maintain optimal stringency during FISH procedures; Critical for probe specificity |
Advanced Technical Considerations
Recent Methodological Innovations
FISH Variants: The development of catalyzed reporter deposition FISH (CARD-FISH) has significantly enhanced signal intensity for targets with low nucleic acid copy numbers [11]. Nucleic Acid Mimics (NAMs), particularly peptide nucleic acids (PNA) and locked nucleic acids (LNA), have emerged as superior alternatives to traditional DNA probes, offering higher affinity, greater specificity, and enhanced resistance to nuclease degradation [14]. For complex microbial communities, combinatorial labeling and spectral imaging FISH (CLASI-FISH) enables simultaneous visualization of multiple microbial taxa, while double labeling of oligonucleotide probes for FISH (DOPE-FISH) provides doubled signal intensity [11].
PCR Advancements: Digital droplet PCR (ddPCR) represents a significant innovation for absolute quantification of target DNA without standard curves, offering particular advantages for detecting rare targets and when PCR inhibition is a concern [85]. Isothermal amplification methods such as loop-mediated isothermal amplification (LAMP) provide alternatives to conventional PCR, enabling rapid detection in resource-limited settings without thermal cycling equipment [90] [85]. In clinical diagnostics, RT-qPCR has largely replaced traditional Nested-PCR as the gold standard for detecting genetic alterations in hematological malignancies due to its superior sensitivity and quantitative capabilities [89].
Application-Specific Recommendations
Clinical Microbiology: For bloodstream infections, PCR demonstrates superior sensitivity (71.7% vs. 39.1% for FISH) and remains unaffected by prior antibiotic administration [86]. However, FISH provides faster results (4-5 hours) compared to conventional blood culture (several days), enabling more timely intervention in septic patients.
Oncology Diagnostics: FISH excels in detecting specific chromosomal translocations with scattered breakpoints, such as t(11;14) in mantle cell lymphoma, where it significantly outperforms genomic PCR (97% vs. 37% detection) [87]. In hematological malignancies, RT-qPCR has demonstrated higher sensitivity than Nested-PCR for detecting fusion genes like BCR::ABL1 and PML::RARA [89].
Food Safety and Authenticity: For species identification in highly processed products like canned tuna, real-time PCR achieves superior detection rates (100%) compared to both multiplex PCR (29%) and DNA barcoding methods (33%) [88]. The method's robustness to DNA fragmentation during processing makes it particularly suitable for such applications.
The choice between FISH and PCR technologies requires careful consideration of application-specific requirements. FISH provides unparalleled spatial context and morphological preservation, making it ideal for localization studies and detecting chromosomal rearrangements with scattered breakpoints. PCR technologies, particularly real-time and digital PCR, offer superior sensitivity and quantification capabilities, crucial for pathogen detection in clinical samples and monitoring minimal residual disease in oncology. Recent advancements in both methodologies, including NAMs for FISH and isothermal amplification for PCR, continue to expand their applications and performance. Researchers should base their selection on the specific requirements of their experimental questions, considering factors such as the need for quantification, spatial information, sensitivity thresholds, and available resources. A complementary approach utilizing both techniques may provide the most comprehensive analytical solution for complex research questions in microbial detection and beyond.
Genomic screening technologies are cornerstone tools for detecting genetic abnormalities in both clinical diagnostics and fundamental research. Fluorescence in situ hybridization (FISH) is a powerful cytogenetic technique that enables the visualization of specific DNA sequences within chromosomes, cells, or tissues [51] [29]. In parallel, microarray-based comparative genomic hybridization (array CGH or aCGH) provides a platform for genome-wide scanning to detect copy number variations (CNVs) with high resolution [91] [92]. This application note provides a detailed comparison of these two pivotal technologies, framed within the context of microbial detection research, summarizing their capabilities, methodologies, and applications to guide researchers and scientists in assay selection and implementation.
Fundamental Principles
FISH is a targeted technique based on the hybridization of fluorescently labeled nucleic acid probes to complementary target DNA sequences within metaphase chromosomes or interphase nuclei [51] [29]. The hybridization is then visualized using fluorescence microscopy, allowing for the direct localization of specific genetic loci, entire chromosomes, or microbial taxa [93]. The key steps involve probe labeling, denaturation of target and probe DNA, hybridization, and fluorescence microscopy analysis [29].
Array CGH is a comprehensive, whole-genome technique that compares the DNA content between a test (patient) genome and a normal (reference) genome [91] [92]. The two genomes are differentially labeled with distinct fluorophores, mixed, and co-hybridized competitively to a microarray slide containing thousands of immobilized DNA probes (e.g., oligonucleotides, BACs) [91] [94]. The resulting fluorescence ratio at each probe location indicates relative copy number—regions of gain or loss—in the test genome compared to the reference [92].
Comparative Capabilities
The table below summarizes the core technical characteristics and screening capabilities of FISH and array CGH.
Table 1: Comparative Overview of FISH and Microarray CGH
| Parameter | Fluorescence In Situ Hybridization (FISH) | Microarray CGH (aCGH) |
|---|---|---|
| Basic Principle | Targeted hybridization visualized via microscopy [29] | Genome-wide, competitive hybridization on an array [91] [92] |
| Typical Resolution | Limited by microscopy; generally >50 kb [51] | High; determined by probe density, can detect changes as small as 10 kb [92] [94] |
| Screening Scope | Targeted analysis of specific, pre-defined regions [51] [95] | Whole-genome, hypothesis-free screening [91] [92] |
| Throughput | Lower throughput; one to a few probes per experiment [91] | High throughput; thousands of loci assessed in a single assay [91] [92] |
| Cell Requirement | Requires metaphase chromosomes or intact interphase nuclei [51] | Requires only extracted genomic DNA [91] |
| Key Applications | Detecting aneuploidies, translocations, microdeletions, gene amplifications, and microbial identification [51] [93] [95] | Identifying submicroscopic copy number variations (CNVs) and genomic imbalances on a genome-wide scale [91] [92] [94] |
| Turnaround Time | ~1-3 days [29] | Several days to a week, including data analysis |
| Information Obtained | Provides spatial genomic context and information about chromosomal morphology [51] | Provides a high-resolution map of copy number changes, but no information on chromosomal structure or balanced rearrangements [91] |
Experimental Protocols
Detailed FISH Protocol for Microbial Detection
The following protocol is adapted for the identification and quantification of microorganisms in complex environmental samples, such as manure or soil [93].
Workflow Overview:
Materials & Reagents:
- Sample: Swine manure, soil, or other environmental samples [93].
- Fixatives: 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) or 100% ethanol [93].
- Buffers: 1× PBS (130 mM NaCl, 10 mM sodium phosphate; pH 7.2); 0.1% sodium pyrophosphate (NaPPi) buffer for soil dispersion [93].
- FISH Probes: Fluorescently labeled oligonucleotide probes (e.g., 6-FAM, Alexa488). Example: S-D-Bact-0338-a-A-18 for general bacteria detection [93].
- Hybridization Solution: Contains formamide, salts, and detergents to facilitate specific probe binding [93].
- Wash Solutions: 0.4x SSC and 2x SSC with 0.05% Tween-20 [29].
- Counterstain: 4′,6-diamidino-2-phenylindole (DAPI) at 1 μg/mL [93].
- Equipment: Fluorescence microscope with appropriate filter sets, sonicator, water bath or automated hybridization station (e.g., ThermoBrite) [93] [29].
Step-by-Step Methodology:
Sample Fixation and Dispersion:
- Fix samples in 4% PFA or 100% ethanol for 2 hours on ice to preserve cellular morphology and nucleic acids [93].
- Wash fixed samples three times in PBS.
- Resuspend the fixed sample in a PBS-ethanol mixture (1:1) and store at -20°C.
- Critical Step: To reduce interference from cell aggregates, dilute the fixed sample (e.g., 1:10 for manure, 1:100 for soil) and sonicate. For example, sonicate 10 μL of diluted sample in 2 mL of buffer with a 5-second pulse at 250 W [93].
Slide Preparation:
- Filter the dispersed sample through a black 0.22 μm polycarbonate membrane.
- Transfer cells from the membrane onto a gelatin-coated microscope slide by pressing the filter onto the slide for at least 10 seconds [93].
- Prepare slides fresh on the day of the FISH assay.
Probe Hybridization:
- Apply 15 μL of hybridization solution containing 1 μL (50 ng/μL) of the fluorescent probe to the sample area on the slide [93].
- Place a coverslip and seal the edges with rubber glue to prevent evaporation.
- Denaturation: Heat the slide at 75°C ± 1°C for 2-5 minutes on a hotplate or automated unit to separate DNA strands [29].
- Hybridization: Incubate the slide at 37°C ± 1°C in a humid, dark chamber for a defined period (typically overnight) to allow the probe to bind to its target [93] [29].
Post-Hybridization Washes:
- Remove the coverslip and wash the slide twice to remove unbound and non-specifically bound probe [29]:
- First wash: In 0.4x SSC at 72°C for 2 minutes.
- Second wash: In 2x SSC with 0.05% Tween-20 at room temperature for 30 seconds.
- Air-dry the slide in the dark.
- Apply a DAPI counterstain (e.g., 1 μg/mL for 5 minutes) to visualize all cell nuclei, then rinse and mount [93].
- Remove the coverslip and wash the slide twice to remove unbound and non-specifically bound probe [29]:
Microscopy and Image Analysis:
- Analyze the slide using a fluorescence microscope equipped with DAPI, FITC, and other relevant filter sets [29].
- Acquire images from multiple random fields.
- Automated Image Analysis (Recommended): For complex samples, use automated image analysis software. One robust method involves:
- Detecting cell locations from DAPI micrographs.
- Extracting maximum and mean fluorescence intensities for each cell from the corresponding FISH images.
- Classifying cells as target (positive) or non-target (negative) using Fuzzy c-means (FCM) clustering on the intensity data, which is more reliable than fixed thresholds for samples with variable backgrounds [93].
- Count a minimum of 100-200 interphase cells for reliable quantification [93] [29].
Workflow Overview:
Step-by-Step Methodology:
- DNA Extraction: Extract high-quality genomic DNA from the test sample (e.g., microbial culture, patient blood) and a normal reference sample [91] [92].
- Fluorescent Labeling: Label the test DNA with one fluorophore (e.g., Cy5) and the reference DNA with another (e.g., Cy3) using enzymatic methods [91] [94].
- Hybridization: Mix equal quantities of labeled test and reference DNA, denature the mixture, and apply it to the microarray. Incubate the array to allow competitive hybridization to the immobilized probes [91] [92].
- Washing and Scanning: Wash the array to remove non-specifically bound DNA. Scan the array using a digital imaging system to quantify the fluorescence intensity of each fluorophore at every probe spot [92] [94].
- Data Analysis: Calculate the fluorescence ratio (test/reference) for each probe. A ratio of 1 indicates normal copy number, a ratio >1 indicates gain/amplification, and a ratio <1 indicates loss/deletion in the test genome [91] [92]. Bioinformatic tools are used to visualize and interpret these ratios across the genome.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagent Solutions for FISH and Array CGH
| Item | Function/Application | Examples / Notes |
|---|---|---|
| Oligonucleotide FISH Probes | Target-specific detection of DNA or RNA sequences in cells. | 16S rRNA-targeted probes for microbial identification (e.g., S-D-Bact-0338-a-A-18) [93]. |
| Labeling Systems (Indirect) | Incorporate haptens into probes for subsequent detection. | Biotin, Digoxigenin, or Dinitrophenol. Detected with fluorescently labeled affinity reagents (e.g., avidin, antibodies) [51]. |
| Labeling Systems (Direct) | Incorporate fluorophores directly into probes. | Fluorescein (FITC), Rhodamine, Texas Red, Cy dyes (Cy3, Cy5) [51]. Eliminates need for detection steps. |
| Chromosomal Microarrays | Solid support for genome-wide copy number analysis. | Available in whole-genome or targeted designs; probes can be oligonucleotides, cDNAs, or BACs [91] [92]. |
| Counterstains | Visualize cell nuclei or chromosomes. | DAPI (4′,6-diamidino-2-phenylindole) [93] [29]. |
| Hybridization Buffers & Wash Kits | Create optimal conditions for specific probe binding and remove non-specific signal. | Commercial kits with standardized SSC/SDS-based buffers are recommended for assay consistency [29]. |
FISH and array CGH are complementary technologies with distinct strengths in genomic screening. FISH remains the gold standard for targeted analysis, providing spatial context and is invaluable for confirming specific abnormalities, including in microbial ecology [93] [95] [96]. In contrast, array CGH offers a powerful, high-resolution, genome-wide screening tool for discovering novel copy number variations without prior hypothesis [91] [92] [94]. The choice between them depends on the research question—whether the goal is to deeply characterize a known target or to unbiasedly screen the entire genome for imbalances. For the most comprehensive analysis, they can be used in tandem, with array CGH identifying novel regions of interest and FISH providing validation and cellular localization.
FISH vs. MALDI-TOF MS and Conventional Culture Methods
Within microbial detection research, Fluorescence In Situ Hybridization (FISH) represents a powerful technique for the direct visualization of microorganisms within their environmental context. This application note situates FISH within the modern microbiologist's toolkit by comparing its capabilities with two other foundational methods: conventional culture and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS). Conventional culture, long considered the "gold standard," provides viability data but is hampered by lengthy turnaround times. MALDI-TOF MS has revolutionized high-throughput identification of cultured isolates, while FISH offers unparalleled spatial resolution for ecological studies. The selection of an appropriate method hinges on the specific research question, whether it is rapid pathogen identification, the study of microbial community structure, or the determination of microbial viability and function. This document provides a detailed technical comparison and protocols to guide researchers in applying these techniques effectively.
Technical Comparison of Methods
The following table summarizes the core characteristics of FISH, MALDI-TOF MS, and conventional culture methods, highlighting their respective advantages and limitations.
Table 1: Technical Comparison of FISH, MALDI-TOF MS, and Conventional Culture Methods
| Feature | FISH | MALDI-TOF MS | Conventional Culture |
|---|---|---|---|
| Principle | Hybridization of fluorescently labeled nucleic acid probes to ribosomal RNA | Analysis of ribosomal protein spectral profiles by mass spectrometry [97] | Growth on selective and/or differential media |
| Turnaround Time | 2 - 8 hours (post-sample fixation) | Minutes per sample after pure colony isolation [97] | 24 hours to several days/weeks |
| Sensitivity | High with signal amplification (e.g., CARD-FISH); depends on probe design | Requires ~10^4 - 10^6 CFU/mL for reliable detection [97] | High for viable/cultivable organisms |
| Key Advantage | Spatial resolution, morphology, and viability assessment (with viability probes) | High-throughput, cost-effective, and excellent for species-level ID of cultured isolates [97] | Gold standard for viability; allows antibiotic susceptibility testing (AST) |
| Primary Limitation | Requires prior knowledge of target for probe design; autofluorescence | Limited utility for direct sample analysis; requires protein extraction; database-dependent [97] | Only detects cultivable organisms (<1%); slow |
| Typical Application | Microbial ecology, biofilm studies, pathogen localization in tissues | Rapid identification of bacteria and yeast from pure cultures in clinical diagnostics [97] | Isolation, viability testing, and AST |
Detailed Experimental Protocols
Protocol: FISH for Environmental Biofilm Samples
This protocol outlines the steps for detecting specific microorganisms within a biofilm using FISH.
Sample Fixation:
- Immerse the biofilm sample (e.g., on a surface or filter) in 4% paraformaldehyde (in 1x PBS) for 4 hours at 4°C.
- Wash the fixed sample three times with 1x PBS.
- Dehydrate by successive 5-minute washes in 50%, 80%, and 96% ethanol. Air dry.
Hybridization:
- Prepare hybridization buffer (0.9 M NaCl, 20 mM Tris/HCl [pH 7.2], 0.01% SDS, and formamide at a concentration determined empirically for probe stringency).
- Apply hybridization buffer containing the species-specific fluorescent probe (e.g., 5 ng/μL) to the sample.
- Incubate in a dark, humidified chamber at 46°C for 1.5-3 hours.
Washing:
- Prepare pre-warmed washing buffer (20 mM Tris/HCl [pH 7.2], 0.01% SDS, 5 mM EDTA, and NaCl concentration based on formamide used).
- Remove the hybridization buffer and wash the sample in pre-warmed washing buffer for 15-20 minutes at 48°C.
Counterstaining and Microscopy:
- Rinse briefly with distilled water and air dry in the dark.
- Apply an antifading mounting medium containing DAPI (4',6-diamidino-2-phenylindole) to stain all microbial DNA.
- Analyze using epifluorescence or confocal laser scanning microscopy with appropriate filter sets.
Protocol: MALDI-TOF MS for Microbial Identification from a Pure Culture
This protocol details the direct transfer method for identifying a bacterial or yeast isolate using MALDI-TOF MS [97].
Sample Preparation (Direct Transfer):
- Using a sterile loop, pick a single well-isolated colony from a fresh (18-24 hour) culture plate.
- Smear the biomass thinly as a uniform layer onto a spot of a polished steel MALDI target plate.
- Overlay the smear with 1 μL of matrix solution (e.g., α-cyano-4-hydroxycinnamic acid (α-CHCA) in 50% acetonitrile and 2.5% trifluoroacetic acid) [97].
- Allow the spot to air dry completely at room temperature.
Protein Extraction (Alternative Method, for difficult taxa):
- If the direct transfer fails, a full protein extraction is performed.
- Transfer biomass to a microcentrifuge tube containing 300 μL of deionized water and 900 μL of absolute ethanol. Vortex and centrifuge.
- Dry the pellet and reconstitute with 25-50 μL of 70% formic acid. Mix well.
- Add an equal volume of acetonitrile, mix, and centrifuge.
- Spot 1 μL of the supernatant on the target plate, let it dry, and then overlay with 1 μL of matrix.
Data Acquisition:
- Insert the target plate into the MALDI-TOF MS instrument.
- Acquire mass spectra in linear positive ion mode with a mass range of 2,000 to 20,000 Da.
- The instrument laser fires at multiple points per spot to generate a characteristic protein mass fingerprint.
Data Analysis:
- The acquired spectrum is compared against a reference database (e.g., Bruker MBT or VITEK MS SARAMIS).
- An identification score is generated. A score ≥ 2.000 typically indicates reliable identification to the species level, while a score between 1.700 and 1.999 indicates identification to the genus level [97].
Workflow Visualization
The following diagram illustrates the logical relationship and comparative workflows of the three microbial detection methods, from sample to result.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table lists key reagents and their functions for the core techniques discussed.
Table 2: Essential Research Reagent Solutions for Microbial Detection Methods
| Reagent/Material | Function/Application | Key Considerations |
|---|---|---|
| FISH: Formamide | Component of hybridization buffer used to control stringency. Higher concentrations lower the melting temperature of probe-target duplexes, increasing specificity. | Concentration must be optimized for each probe to balance signal intensity and specificity. |
| FISH: Fluorescently Labeled Nucleic Acid Probes | Binds to complementary ribosomal RNA sequences within fixed cells, enabling detection. | Specificity is determined by probe sequence. Fluorophore choice (e.g., Cy3, FITC) must match microscope filters. |
| MALDI-TOF MS: α-CHCA Matrix | Organic acid that absorbs laser energy, aiding in desorption and ionization of microbial proteins [97]. | Promotes soft ionization of proteins. Must be prepared fresh in solvents like acetonitrile and TFA. |
| MALDI-TOF MS: Bacterial Test Standard (BTS) | A calibrated standard of known proteins used for instrument calibration and mass accuracy verification. | Essential for ensuring reproducible and reliable spectral acquisition across runs. |
| Conventional Culture: Selective Media (e.g., MacConkey Agar) | Supports growth of specific microbial groups while inhibiting others based on chemical additives. | Critical for isolating specific pathogens from complex samples like stool or sputum. |
| General: Ethanol Series (50%, 80%, 96%) | Used for sample dehydration in FISH protocols and in protein extraction for MALDI-TOF MS [98] [97]. | Prevents sample degradation and prepares specimens for subsequent staining or extraction steps. |
Identifying Clinical Scenarios Where FISH is the Preferred Method
Fluorescence in situ hybridization (FISH) represents a critical molecular cytogenetic technique that provides unique advantages for detecting specific chromosomal abnormalities and genetic sequences within cellular contexts. Despite the emergence of various molecular diagnostic platforms, FISH maintains its status as the preferred or "gold standard" method in numerous clinical scenarios due to its unique capability to provide spatial information, high specificity, and capacity to detect cryptic genetic alterations that evade conventional diagnostic methods [79]. This technique employs fluorochrome-labeled DNA probes designed to hybridize with complementary chromosomal sequences, enabling visualization of specific genetic targets through fluorescence microscopy [52].
The enduring clinical value of FISH stems from its ability to bridge the gap between conventional cytogenetics and molecular genetic testing, offering both positional information and copy number assessment of specific genomic regions [52]. For microbial detection and various oncology applications, FISH provides the distinct advantage of visualizing pathogens or genetic abnormalities directly within tissue contexts, preserving morphological relationships that are lost in homogenized sample analyses [99]. This application note delineates specific clinical scenarios where FISH demonstrates superior performance compared to alternative diagnostic modalities and provides detailed experimental protocols for its implementation in microbial detection research.
Clinical Scenarios Favoring FISH Implementation
Detection of Cryptic Chromosomal Abnormalities in Hematologic Malignancies
FISH proves indispensable for identifying chromosome abnormalities with well-documented frequencies of false-negative results from conventional cytogenetics, particularly when clinical and pathological findings suggest specific genetic anomalies [79]. The World Health Organization classification of hematopoietic and lymphoid tumors emphasizes the importance of these chromosomal changes for accurate diagnosis, treatment selection, and therapy monitoring [79].
Table 1: FISH Applications in Hematologic Malignancies with Cryptic Abnormalities
| Malignancy | Genetic Targets | Probe Type | Clinical Utility | False-Negative Rate with Conventional Cytogenetics |
|---|---|---|---|---|
| Acute Lymphoblastic Leukemia (ALL) | ETV6(TEL)/RUNX1(AML1), MLL rearrangements | Break-apart, dual-fusion | Risk stratification, therapeutic decisions | 50-100% for ETV6-RUNX1 [79] |
| Chronic Lymphocytic Leukemia/SLL | del(13q14), del(11q22)/ATM, del(17p13)/TP53, +12 | Locus-specific identifiers | Patient stratification into prognostic categories | High (mature B-cells often show poor in vitro growth) [79] |
| Plasma Cell Myeloma | del(13q14), t(4;14)/FGFR3-IGH, trisomies 7,9,15 | Locus-specific, centromeric | Prognostic information | Routine cytogenetics often normal due to limited plasma cell infiltration [79] [100] |
| Acute Myeloid Leukemia (AML) | CBFA2T1(ETO)/RUNX1, PML/RARA, CBFB/MYH11 | Dual-fusion, break-apart | Diagnosis, treatment selection | ~5-10% for core abnormalities [79] |
| Chronic Myelogenous Leukemia (CML) | BCR/ABL1 | Dual-fusion | Diagnosis, monitoring response to therapy | ~5-10% [79] |
In acute promyelocytic leukemia with suspected PML/RARA rearrangement, FISH or other rapid methodologies should be performed with same-day or next-day turnaround to facilitate timely treatment with all-trans-retinoic acid [79]. Similarly, FISH serves as a preferred method when conventional cytogenetics fails or when metaphase cells are unavailable for analysis, such as with formalin-fixed, paraffin-embedded tissue specimens [79] [52].
Solid Tumor Biomarker Detection with Therapeutic Implications
In solid tumor diagnostics, FISH maintains "gold standard" status for detecting several critical biomarkers that guide targeted therapy decisions. This technique is particularly valuable when genetic heterogeneity or breakpoint variability compromises the effectiveness of PCR-based assays [79] [101].
Table 2: FISH as Gold Standard in Solid Tumor Biomarker Detection
| Tumor Type | Genetic Alteration | Probe Type | Therapeutic Implications | Clinical Guidelines |
|---|---|---|---|---|
| Breast Cancer | HER2 amplification | Locus-specific identifier (HER2/CEN17) | HER2-targeted therapies (trastuzumab, T-DM1, DS-8201) | CSCO: IHC 2+ requires FISH confirmation [101] |
| Gastric Cancer | HER2 amplification | Locus-specific identifier (HER2/CEN17) | HER2-targeted therapies | CSCO: IHC 2+ requires FISH confirmation [101] |
| Non-Small Cell Lung Cancer | ALK fusion | Break-apart | ALK inhibitors (alectinib, crizotinib, ceritinib) | FISH is ALK fusion detection gold standard [101] |
| Breast Cancer | TOP2A amplification/deletion | Locus-specific identifier | Anthracycline sensitivity/resistance | TOP2A amplifications predict benefit from CEF regimen [101] |
| Soft Tissue and Bone Tumors | Gene rearrangements (e.g., SS18-SSX) | Break-apart | Diagnostic classification | Detection of fusion genes superior to break-apart in some cases [101] |
For HER2 assessment in breast and gastric cancers, the 2022 CSCO guidelines recommend FISH testing for cases showing equivocal (2+) immunohistochemistry results [101]. Similarly, in non-small cell lung cancer, ALK rearrangement detection via FISH remains the gold standard for identifying patients who may benefit from ALK inhibitor therapies, despite the availability of alternative detection methods [101].
Microbial Detection with Spatial Context Preservation
In microbial detection research, FISH offers unique advantages for identifying and localizing pathogens within complex samples while preserving spatial relationships. This capability proves particularly valuable when investigating host-pathogen interactions, biofilm-associated infections, and difficult-to-culture microorganisms [99].
Table 3: FISH Applications in Microbial Detection and Characterization
| Application Area | Target | Probe Type | Advantages Over Alternative Methods | Sample Types |
|---|---|---|---|---|
| General Microbial Detection | 16S/18S rRNA | Species-specific | Direct visualization, preservation of spatial relationships, viability assessment | Cell cultures, tissue sections, microbial populations [99] |
| Cervical Cancer Screening | TERC gene amplification | Locus-specific identifier | Distinguishes high-grade (CIN2+) from low-grade (CIN1-) lesions | Cervical cytology specimens [101] |
| Urinary Tract Malignancies | Chromosomes 3,7,17, p16 | Centromeric, locus-specific | Non-invasive early diagnosis, monitoring recurrence | Urine specimens [101] |
| Biofilm-associated Infections | Species-specific rRNA sequences | Custom-designed | In situ analysis within biofilm matrix | Biofilm samples, tissue sections [99] |
| Complex Microbiomes | Phylogenetic marker genes | Multi-color panels | Simultaneous detection of multiple taxa | Environmental samples, host-associated microbiomes [99] |
For cervical cancer screening, FISH detection of TERC gene amplification demonstrates superior performance compared to conventional methods, with the ability to distinguish high-grade (CIN2+) from low-grade (CIN1-) lesions with sensitivity and specificity exceeding 90% [101]. This represents a significant improvement over morphological cytology alone, which shows sensitivity of 55-80% and is subject to interpretive variability [101].
Comprehensive FISH Experimental Protocol for Microbial Detection
Sample Preparation and Fixation
Proper sample preparation is critical for successful FISH experiments. For microbial detection, various sample types can be utilized, including cell cultures, tissue sections, and environmental samples [99].
- Sample Collection: Obtain microbial cells through culture, tissue sampling, or environmental collection. For biofilm samples, preserve structural integrity during collection.
- Fixation: Fix samples promptly to maintain cellular morphology and nucleic acid integrity. Use appropriate fixatives such as 4% paraformaldehyde for 1-4 hours at 4°C [99].
- Permeabilization: Treat fixed samples with permeabilization agents to facilitate probe entry. For Gram-negative bacteria, use lysozyme (0.1-10 mg/mL); for Gram-positive bacteria, use lysozyme combined with labial protease or proteinase K [99].
- Slide Preparation: Apply fixed and permeabilized samples to charged glass slides. Air dry and dehydrate through ethanol series (50%, 80%, 96%) for 3 minutes each [99].
Probe Design and Labeling
Probe design fundamentally determines FISH specificity and sensitivity. For microbial detection, ribosomal RNA (16S/18S) often serves as the target due to its abundance and phylogenetic significance [99].
- Target Selection: Identify unique target sequences specific to the microbe of interest using genomic databases. The minimum target length should be 18-30 nucleotides for optimal specificity and accessibility [99].
- Probe Design: Design oligonucleotide probes with appropriate melting temperature (Tm ≈ 50-60°C). Avoid cross-hybridization to non-target sequences by performing thorough database searches [99].
- Fluorescent Labeling: Select fluorophores based on available microscope filters and experimental design. Common options include:
- FITC (Excitation: 490nm, Emission: 520nm) - Green fluorescence
- Texas Red (Excitation: 596nm, Emission: 615nm) - Red fluorescence
- Cy5 (Excitation: 649nm, Emission: 670nm) - Far-red fluorescence [99]
- Control Probes: Include appropriate controls:
- Positive control: Universal bacterial probe (e.g., EUB338)
- Negative control: Non-targeting probe with scrambled sequence [99]
Hybridization and Post-Hybridization Washes
The hybridization process represents the core FISH procedure where probes specifically bind to complementary target sequences.
- Hybridization Mixture Preparation: Prepare hybridization buffer containing appropriate salts, formamide, detergent, and blocking agents. The formamide concentration should be optimized based on probe Tm (typically 0-50%).
- Denaturation: Apply probe mixture to prepared samples and denature simultaneously at 75-80°C for 5-10 minutes [69].
- Hybridization: Incubate slides in a humidified chamber at appropriate hybridization temperature (typically 46°C) for 1.5-12 hours, depending on probe characteristics and target abundance [99].
- Post-Hybridization Washes: Remove non-specifically bound probes through stringent washing:
- Wash with prewarmed hybridization buffer for 10-20 minutes at 48°C
- Subsequent wash with phosphate-buffered saline or Tris-buffered saline [99]
Detection and Microscopic Visualization
Proper detection and visualization are essential for accurate interpretation of FISH results.
- Counterstaining: Apply appropriate counterstains to visualize total cells. DAPI (4',6-diamidino-2-phenylindole) at 1 μg/mL effectively stains DNA and is compatible with most fluorophores [52] [99].
- Mounting: Mount samples in anti-fading mounting medium to preserve fluorescence signal during microscopy and storage.
- Microscopy: Examine slides using an epifluorescence microscope equipped with appropriate filter sets for the fluorophores used. For multicolor FISH, ensure minimal bleed-through between channels [52] [99].
- Image Acquisition: Capture digital images using a cooled CCD camera. For quantitative analysis, maintain consistent exposure settings across compared samples.
- Analysis: Evaluate samples for specific fluorescence signals. For microbial detection, classify cells as target-positive when displaying bright, morphologically appropriate fluorescence signals exceeding background levels [99].
Figure 1: Microbial FISH Experimental Workflow. This diagram outlines the key procedural stages in fluorescence in situ hybridization for microbial detection, from sample preparation through final analysis.
Research Reagent Solutions for Microbial FISH
Successful FISH implementation requires carefully selected reagents and probes optimized for specific research applications. The following table details essential components for microbial FISH experiments.
Table 4: Essential Research Reagents for Microbial FISH Detection
| Reagent Category | Specific Examples | Function | Application Notes |
|---|---|---|---|
| Fixatives | Paraformaldehyde (4%), Ethanol:Acetic Acid (3:1) | Preserve cellular morphology and nucleic acid integrity | Paraformaldehyde preferred for microbial samples; fixation time varies by sample thickness [99] |
| Permeabilization Agents | Lysozyme, Proteinase K, Labial Protease | Enable probe access to intracellular targets | Gram-positive bacteria require more aggressive permeabilization than Gram-negative [99] |
| Fluorescent Probes | 16S/18S rRNA-targeted oligonucleotides | Hybridize to specific microbial sequences | Design targeting variable regions for species-level identification [99] |
| Fluorophores | FITC, Texas Red, Cy5, Cy3 | Signal generation | Select fluorophores based on available filter sets; consider photostability [99] [102] |
| Hybridization Buffers | Formamide, SSC, Dextran Sulfate, Denhardt's Solution | Create optimal stringency conditions | Formamide concentration determines hybridization stringency [99] |
| Counterstains | DAPI, Propidium Iodide | Visualize total cellular content | DAPI compatible with most fluorophores; use at 1 μg/mL [52] [99] |
| Mounting Media | Anti-fade formulations (e.g., Vectashield) | Preserve fluorescence signal | Essential for quantitative analysis and image documentation [99] |
| Blocking Agents | Salmon Sperm DNA, tRNA, BSA | Reduce non-specific binding | Particularly important for complex samples with high background [99] |
Commercial probe suppliers such as Abnova offer comprehensive FISH probe systems including Gene FISH probes for amplification/deletion analysis, Split FISH probes for gene rearrangement detection, and translocation FISH probes for specific fusion events [102]. For microbial applications, companies like TinyGene provide custom probe design services targeting specific microbial sequences with appropriate fluorophore conjugations [99].
Advanced Technical Considerations
Probe Binding Mechanism and Signal Detection
Understanding the molecular mechanism of FISH probe binding enables researchers to optimize experimental conditions and troubleshoot effectively. The process involves sequential molecular interactions that ultimately yield detectable fluorescence signals specifically localized to target sequences.
Figure 2: FISH Probe Binding and Detection Mechanism. This diagram illustrates the sequential process of specific probe-target hybridization and signal detection in FISH applications.
Methodological Advantages and Limitations
While FISH offers numerous advantages for specific clinical and research scenarios, researchers must also acknowledge its limitations to appropriately position it within the methodological landscape.
Key Advantages:
- Spatial Context Preservation: FISH uniquely maintains the spatial organization of microbial communities within their native environments, enabling investigation of spatial relationships in biofilms and host-microbe interactions [99].
- High Specificity and Sensitivity: Well-designed probes can distinguish closely related microbial species with single-nucleotide specificity under optimized conditions [99] [102].
- Direct Visualization: The technique provides direct morphological correlation, allowing researchers to associate genetic signals with specific cellular structures [101] [99].
- Adaptability to Diverse Samples: FISH can be applied to various sample types including cultured cells, tissue sections, cytological preparations, and environmental samples without requiring nucleic acid extraction [79] [52] [99].
Inherent Limitations:
- Targeted Approach: Unlike sequencing-based methods, FISH requires prior knowledge of target sequences, making it unsuitable for discovery-based applications [52].
- Technical Complexity: The multi-step process demands expertise in sample preparation, hybridization optimization, and microscopic analysis [52].
- Limited Multiplexing Capacity: While multi-color FISH is possible, the number of simultaneously detectable targets is constrained by spectral overlap of available fluorophores [52].
- Semi-Quantitative Nature: While FISH provides some quantitative information, its dynamic range for expression analysis is limited compared to PCR-based methods [79] [52].
Troubleshooting Common Experimental Challenges
Successful FISH implementation requires anticipation and resolution of common technical challenges:
- High Background Fluorescence: Optimize permeabilization conditions, increase post-hybridization wash stringency, incorporate appropriate blocking agents, and titrate probe concentrations to minimize non-specific binding [99].
- Weak or Absent Signal: Verify probe quality and concentration, check hybridization efficiency through positive controls, optimize fixation conditions to preserve target accessibility, and confirm fluorophore integrity [99].
- Autofluorescence Issues: Incorporate Sudan Black B treatment to reduce autofluorescence, utilize fluorophores with emission spectra distinct from autofluorescence, or employ time-resolved fluorescence imaging [99].
- Sample Morphology Degradation: Optimize fixation protocol, avoid over-digestion during permeabilization, and use adhesive-coated slides to prevent sample loss [99].
FISH remains the preferred methodological approach in numerous clinical and research scenarios where spatial context, high specificity, and direct visualization are paramount. In hematologic malignancies, FISH detects cryptic abnormalities that inform prognosis and therapeutic decisions [79]. For solid tumors, it provides gold-standard assessment of therapeutically relevant biomarkers such as HER2 and ALK [101]. In microbial detection, FISH enables pathogen identification within complex samples while preserving structural relationships [99].
The continuing evolution of FISH technologies, including advanced multiplexing approaches and enhanced signal detection systems, promises to expand its applications in both clinical diagnostics and fundamental research. By following standardized protocols while incorporating appropriate controls and validation procedures, researchers can leverage the full potential of FISH for sophisticated microbial detection and characterization.
Integrating FISH with Other Techniques (e.g., Flow Cytometry, Immunohistochemistry)
Fluorescence in situ hybridization (FISH) has established itself as a powerful technique in microbial detection research, allowing for the specific identification and spatial localization of microorganisms within complex samples. However, the integration of FISH with other established methodologies significantly expands its analytical capabilities, creating synergistic tools that provide multifaceted data from a single experiment. These hybrid approaches are revolutionizing microbial ecology, clinical diagnostics, and drug development research by simultaneously revealing microbial identity, spatial distribution, functional activity, and host-microbe interactions. This article details the practical application notes and protocols for key integrated FISH techniques, providing researchers with the necessary framework to implement these advanced methods in their investigative workflows. By combining FISH with flow cytometry, immunohistochemistry, and various molecular techniques, scientists can transcend the limitations of individual methods, achieving a more comprehensive understanding of microbial systems in their native contexts.
Integrated FISH Techniques: Application Notes and Protocols
The convergence of FISH with other technologies has created a sophisticated toolkit for addressing complex research questions. Below are detailed protocols and application considerations for the most impactful integrations.
Flow-FISH: Combining FISH with Flow Cytometry
Application Notes: Flow-FISH integrates the cellular localization capability of FISH with the high-throughput quantitative power of flow cytometry. This combination is particularly valuable for screening applications where statistical analysis of large cell populations is required. A prominent application is the measurement of telomere length in leukocyte subpopulations for the diagnosis of cryptic dyskeratosis congenita (DKC) in adult patients. Studies have demonstrated that Flow-FISH provides superior specificity and a lower false-positive rate compared to molecular methods like monochrome multiplex quantitative PCR (MM-qPCR), especially when using the 10% age-adjusted threshold for telomere length, making it the recommended gold standard for such clinical screenings [103].
Protocol:
- Sample Preparation: Isolate peripheral blood mononuclear cells (PBMCs) from fresh whole blood using density gradient centrifugation.
- Probe Hybridization:
- Resuspend the cell pellet in a hybridization mixture containing the fluorescently labeled (e.g., FITC) telomere-specific peptide nucleic acid (PNA) probe.
- Denature the cellular DNA and the probe simultaneously at 80-85°C for 10 minutes.
- Hybridize in the dark at room temperature for a minimum of 2 hours or overnight for enhanced signal intensity.
- Post-Hybridization Washes: Perform a series of stringent washes to remove unbound and nonspecifically bound probe.
- Flow Cytometry Analysis:
- Counterstain cells with a DNA-specific dye like DAPI to gate on intact, nucleated cells.
- Analyze the cells on a flow cytometer equipped with a laser suitable for exciting the fluorescent probe.
- For telomere length quantification, measure the mean fluorescence intensity (MFI) of the telomere probe in the target cell population (e.g., lymphocytes) and normalize it to the MFI of a control sample with known telomere length.
- Fluorescence-Activated Cell Sorting (FACS): As an optional subsequent step, the hybridized cells can be sorted based on their fluorescence signal for downstream molecular analyses or culture [103].
Immuno-FISH: Combining FISH with Immunohistochemistry (IHC)
Application Notes: The combination of FISH and IHC, often termed Immuno-FISH or IHC-FISH, allows for the correlative analysis of nucleic acid markers and protein expression within the same tissue section, preserving the critical spatial context. This is invaluable in cancer research for linking genetic aberrations with protein-based phenotypic markers and in microbiome research for associating specific microbes with host immune response markers. The primary challenge is maintaining the integrity of the protein epitopes for IHC while ensuring sufficient DNA/RNA accessibility for the FISH probe, often requiring careful optimization of the fixation and permeabilization steps [104] [105].
Protocol:
- Tissue Preparation: Use formalin-fixed, paraffin-embedded (FFPE) tissue sections mounted on charged slides. Deparaffinize and rehydrate the sections through a series of xylene and ethanol washes [104].
- Antigen Retrieval: Perform heat-induced or protease-induced epitope retrieval to unmask protein epitopes for IHC. The method and duration must be optimized for the specific primary antibody to be used [104].
- Immunohistochemistry (First Assay):
- Block endogenous peroxidases and nonspecific protein binding sites.
- Incubate with the primary antibody targeting the protein of interest.
- Detect the antibody using an enzymatic system (e.g., HRP) with a chromogenic substrate like DAB, which yields a stable, non-fluorescent precipitate.
- FISH (Second Assay):
- Post-fix the IHC-stained slides to preserve the antibody complex.
- Apply the fluorescently labeled nucleic acid probe to the tissue section.
- Co-denature the specimen and probe, typically at 75-80°C, and allow for hybridization overnight in a humidified chamber.
- Perform stringent washes to remove excess probe.
- Counterstaining and Mounting: Counterstain with DAPI to visualize nuclei and mount with an anti-fade mounting medium.
- Imaging: Acquire images using a brightfield microscope to document the IHC signal, followed by fluorescence microscopy with appropriate filter sets to capture the FISH and DAPI signals. Image overlay software is used to merge the signals from both assays [104] [105].
FISH with Catalytic Amplification: CARD-FISH
Application Notes: Catalyzed Reporter Deposition FISH (CARD-FISH) uses horseradish peroxidase (HRP)-labeled oligonucleotide probes and tyramide signal amplification to dramatically increase the fluorescence intensity of the FISH signal. This is particularly crucial for the detection of microorganisms with low ribosomal RNA content, a common issue in environmental samples, slow-growing bacteria, or in samples with high background fluorescence. CARD-FISH is widely used in aquatic and soil microbiology for detecting and quantifying microbial community members [105].
Protocol:
- Sample Fixation and Permeabilization: Fix cells or tissue sections with paraformaldehyde. A critical step for CARD-FISH is the permeabilization of the cell wall/membrane to allow the large HRP enzyme to enter the cell. This often requires optimization using lysozyme or other enzymes.
- Hybridization: Hybridize with an HRP-labeled oligonucleotide probe specific to the target rRNA sequence under standard conditions.
- Signal Amplification:
- After stringent washes, incubate the sample with fluorescently labeled tyramide substrates.
- The HRP enzyme catalyzes the deposition of multiple tyramide molecules at the site of the probe binding, resulting in a massively amplified signal.
- Counterstaining and Microscopy: Counterstain with DAPI and analyze via fluorescence microscopy. The significantly brighter signals allow for easier detection and automated counting of microbial cells [105].
Table 1: Comparison of Key Integrated FISH Techniques
| Technique | Primary Application | Key Advantage | Limitation |
|---|---|---|---|
| Flow-FISH [103] | High-throughput telomere length measurement in leukocytes. | Quantitative, population-level data; high statistical power. | Loses spatial context of the sample. |
| Immuno-FISH [104] [105] | Correlating genetic alterations with protein expression in situ. | Preserves spatial relationship between nucleic acids and proteins. | Technical complexity; potential interference between protocols. |
| CARD-FISH [105] | Detecting microbes with low rRNA content in environmental samples. | High sensitivity; significantly amplified signal. | Permeabilization can be challenging; may cause high background. |
| FISH-NanoSIMS [105] | Linking microbial identity with metabolic function at single-cell level. | Provides ultra-high resolution elemental and isotopic data. | Extremely expensive equipment; complex sample preparation. |
| MAR-FISH [105] | Identifying metabolically active microorganisms within communities. | Directly measures substrate uptake/utilisation. | Involves radioactive isotopes; requires specialized safety protocols. |
Research Reagent Solutions and Essential Materials
Successful implementation of integrated FISH techniques relies on a suite of specialized reagents and materials. The table below details the key components and their functions.
Table 2: Essential Research Reagents for Integrated FISH Workflows
| Reagent/Material | Function | Application Notes |
|---|---|---|
| Peptide Nucleic Acid (PNA) Probes [105] | Synthetic probes with a neutral backbone for FISH; improve hybridization affinity and specificity. | Ideal for Flow-FISH and detection of microbes with complex cell walls. Resists enzymatic degradation. |
| Locked Nucleic Acid (LNA) Probes [105] | Modified RNA nucleotides with increased thermal stability; enhance probe binding strength. | Used in NAM-FISH to improve signal intensity and specificity in challenging samples like thick biofilms. |
| Tyramide Signal Amplification Reagents | Enzyme-mediated deposition of fluorescent tyramides for signal amplification. | Core component of CARD-FISH; drastically increases detection sensitivity. |
| VECTASHIELD HardSet with DAPI [106] | Antifade mounting medium with nuclear counterstain. | Prevents photobleaching; contains DAPI for nuclear visualization. HardSet formulation reduces handling damage. |
| IntelliFISH Hybridization Buffer [106] | Proprietary buffer for FISH hybridization. | Enables significant reduction in hybridization time (e.g., from 18 hours to 4 hours) while maintaining strong, distinct signals. |
| HRP-Labeled Probes [105] | Oligonucleotide probes conjugated to Horseradish Peroxidase enzyme. | Used in CARD-FISH to catalyze the tyramide signal amplification reaction. |
| Primary Antibodies for IHC | Proteins that bind specifically to target antigens in tissue samples. | The specific choice (e.g., monoclonal vs. polyclonal, host species) is determined by the protein target in Immuno-FISH workflows [104]. |
Workflow and Signaling Pathways
The following diagram illustrates the generalized conceptual workflow for designing and executing a project involving FISH integrated with another technique, highlighting the key decision points and procedural steps.
The integration of FISH with complementary techniques like flow cytometry and immunohistochemistry represents a powerful paradigm in modern biological research. These hybrid methods, as detailed in the application notes and protocols above, enable researchers to move beyond mere detection to achieve a multidimensional understanding of microbial identity, function, and interaction within their natural environments. While each combination presents unique technical challenges, the resultant data—quantifiable, spatially resolved, and functionally annotated—is invaluable for advancing fields from microbial ecology to clinical diagnostics and therapeutic development. As probe design, imaging technologies, and signal amplification strategies continue to evolve, the potential for novel integrations and applications of FISH will undoubtedly expand, further solidifying its role as an indispensable tool in the scientist's arsenal.
Conclusion
FISH remains an indispensable and dynamically evolving tool in microbial detection, uniquely offering culture-free, spatially resolved identification of microorganisms within their native contexts. Its strength lies in the direct visualization of pathogens, the ability to perform multiplex analysis, and the capacity to detect unculturable species. While techniques like PCR offer higher sensitivity for minimal residual disease, FISH provides superior capability for identifying numerical abnormalities and structural rearrangements where breakpoint heterogeneity challenges PCR-based methods. Future directions point toward the increased use of synthetic nucleic acid mimics like PNA for enhanced permeability and specificity, the development of robust FRET-based and cleavable probes for sequential multi-target imaging, and deeper integration with high-throughput and super-resolution technologies. For researchers and clinicians, mastering both the foundational principles and advanced optimization of FISH is crucial for pushing the boundaries of diagnostic microbiology, personalized medicine, and our understanding of complex microbial ecosystems.
References
- 1. Fluorescence in situ hybridization [https://en.wikipedia.org/wiki/Fluorescence_in_situ_hybridization]
- 2. What is fluorescence in situ hybridisation? [https://www.ogt.com/resources/fish-resources-and-support/introduction-to-fish/what-is-fish/]
- 3. Fluorescence In Situ Hybridization (FISH) [https://www.thermofisher.com/sg/en/home/life-science/cell-analysis/cellular-imaging/in-situ-hybridization-ish/fluorescence-in-situ-hybridization-fish.html]
- 4. Fluorescence in situ hybridization protocol for ... [https://www.sciencedirect.com/science/article/pii/S0022282825000240]
- 5. Applicability of live-fluorescence in situ hybridization ... [https://www.sciencedirect.com/science/article/pii/S0038071725002329]
- 6. Fluorescent In Situ Hybridization (FISH) Probe Market Size, ... [https://www.precedenceresearch.com/fluorescent-in-situ-hybridization-probe-market]
- 7. Fluorescent In Situ Hybridization (FISH) Probe Market Size, ... [https://www.strategicmarketresearch.com/market-report/fluorescent-in-situ-hybridization-probe-market]
- 8. Fluorescence In Situ Hybridization (FISH) [https://link.springer.com/book/10.1007/978-1-0716-3766-1]
- 9. U-FISH: a fluorescent spot detector for imaging-based spatial ... [https://genomebiology.biomedcentral.com/articles/10.1186/s13059-025-03736-x]
- 10. Global Fluorescent In Situ Hybridization Probe Market [https://finance.yahoo.com/news/fluorescent-situ-hybridization-fish-probe-161300253.html]
- 11. Application of Fluorescence In Situ Hybridization (FISH) ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9788346/]
- 12. MiniReview Fluorescence in situ hybridisation (FISH) [https://www.sciencedirect.com/science/article/abs/pii/S0378109705002375]
- 13. What are the factors that influence Fluorescence in situ ... [https://www.aatbio.com/resources/faq-frequently-asked-questions/what-are-the-factors-that-influence-fluorescence-in-situ-hybridization-fish-performance]
- 14. The role of Nucleic Acid Mimics (NAMs) on FISH-based ... [https://www.sciencedirect.com/science/article/pii/S0944501322001264]
- 15. Comparison of Different In Situ Hybridization Techniques for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6071121/]
- 16. Mechanistic Approach to the Problem of Hybridization ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC535158/]
- 17. Fluorescence in situ hybridization (FISH) and cell sorting of ... [https://www.nature.com/articles/s41598-019-55049-2]
- 18. A Multicolor Fluorescence in situ Hybridization Approach ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2019.01383/full]
- 19. Spatial profiling of microbial communities by sequential ... [https://www.nature.com/articles/s41467-023-37188-3]
- 20. Automated Design of Probes for rRNA-Targeted Fluorescence ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4135741/]
- 21. The Comparative RNA Web (CRW) Site: an online database ... [https://bmcbioinformatics.biomedcentral.com/articles/10.1186/1471-2105-3-2]
- 22. Learning, visualizing and exploring 16S rRNA structure using ... [https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1009345]
- 23. Fluorescence In Situ Hybridization (FISH) [https://www.genome.gov/genetics-glossary/Fluorescence-In-Situ-Hybridization-FISH]
- 24. Protocol to investigate gene expression heterogeneity in ... [https://www.sciencedirect.com/science/article/pii/S2666166725006185]
- 25. CARD-FISH in the Sequencing Era: Opening a New ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2021.640066/full]
- 26. In situ hybridisation - biomers.net Oligonucleotides [https://www.biomers.net/en/products/dna/in_situ_hybridisation.html]
- 27. Automation of fluorescent in situ hybridization (FISH) ... [https://pubmed.ncbi.nlm.nih.gov/40962484/]
- 28. Fluorescence in Situ hybridization (FISH) [https://www.mpi-bremen.de/en/Fluorescence-in-Situ-hybridization-FISH.html]
- 29. What is fluorescence in situ hybridization? [https://www.ogt.com/ca/resources/fish-resources-and-support/introduction-to-fish/what-is-fish/]
- 30. CN102839208B - 一ç§è§å…‰åŽŸä½æ‚交细胞计数的ç»å¯¹å®šé‡æ–¹æ³• [https://patents.google.com/patent/CN102839208B/zh]
- 31. Fluorescent in situ Hybridization (FISH) [https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/fish-procedure?srsltid=AfmBOor63JjNaxG_zXWxj9as0laOx1ii5i0iOO6-ekReZEuQTpuzzgT6]
- 32. Fluorescence In Situ Hybridization (FISH) [https://www.thermofisher.com/us/en/home/life-science/cell-analysis/cellular-imaging/in-situ-hybridization-ish/fluorescence-in-situ-hybridization-fish.html]
- 33. A quick and simple FISH protocol with hybridization-sensitive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3261739/]
- 34. What are the general steps of fluorescence in situ ... [https://www.aatbio.com/resources/faq-frequently-asked-questions/What-are-the-general-steps-of-fluorescence-in-situ-hybridization-FISH-experiments]
- 35. FFPE tissue preparation and FISH protocol [https://www.ogt.com/resources/fish-resources-and-support/introduction-to-fish/fish-procedures/ffpe-tissue-preparation-and-fish-protocol/]
- 36. FFPE Tissue Preparation and FISH Protocol [https://www.creative-bioarray.com/support/ffpe-tissue-preparation-and-fish-protocol.htm]
- 37. Fluorescence In Situ Hybridization (FISH) Tests for Identifying ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9141552/]
- 38. High-content enhancement strategies for fluorescence in ... [https://www.sciencedirect.com/science/article/abs/pii/S0165993625002225]
- 39. TrueProbes: Quantitative Single-Molecule RNA-FISH ... [https://elifesciences.org/reviewed-preprints/108599]
- 40. Protocol optimization improves the performance of ... [https://www.nature.com/articles/s41598-025-17477-1]
- 41. Fluorescence In Situ Hybridization (FISH) protocol [https://www.creativebiomart.net/resource/principle-protocol-fluorescence-in-situ-hybridization-fish-protocol-342.htm]
- 42. Overview of In Situ Hybridization [https://www.creative-bioarray.com/support/ish-fish.htm]
- 43. 10 Tips for Successful FISH [https://www.enzo.com/note/10-tips-for-successful-fish/]
- 44. Hematology FISH protocol - Post-hybridization washes [https://www.ogt.com/ca/resources/fish-resources-and-support/fish-support/hematology-fish-protocol-post-hybridization-washes/]
- 45. Fast and accurate multi-bacterial identification using ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566324009576]
- 46. Combined fluorescent in situ hybridization for detection of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3779857/]
- 47. Spectral imaging and nucleic acid mimics fluorescence in ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2022.976639/full]
- 48. SABER-FISH – Signal amplification for multiplexed ... [https://www.protocols.io/view/saber-fish-signal-amplification-for-multiplexed-fl-dzp975r6.html]
- 49. Mapping human tissues with highly multiplexed RNA in situ ... [https://www.nature.com/articles/s41467-024-46437-y]
- 50. Development of a Multiplex Fluorescence in Situ ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9293417/]
- 51. Fluorescence In Situ Hybridization (FISH) and Its Applications [https://pmc.ncbi.nlm.nih.gov/articles/PMC7122835/]
- 52. Fluorescent in situ hybridisation (FISH) — Knowledge Hub [https://www.genomicseducation.hee.nhs.uk/genotes/knowledge-hub/fluorescent-in-situ-hybridisation-fish/]
- 53. Reducing your FISH probe validation burden [https://www.ogt.com/us/resources/fish-resources-and-support/fish-resources/reducing-your-fish-probe-validation-burden/]
- 54. Fluorescence In Situ Hybridization Probe Validation for ... [https://pubmed.ncbi.nlm.nih.gov/27910018/]
- 55. Culture-free detection of bacteria from blood for rapid ... [https://www.nature.com/articles/s41746-025-01948-w]
- 56. Rapid diagnosis of bloodstream infections using a culture ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11039749/]
- 57. Protocol for Rapid Fluorescence In Situ Hybridization of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC154512/]
- 58. Improved Fluorescent In Situ Hybridization Method for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1828804/]
- 59. EDTA-FISH: A Simple and Effective Approach to Reduce ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7511785/]
- 60. How do I reduce high background in my FISH assay? [https://www.ogt.com/ca/resources/fish-resources-and-support/fish-resources/optimize-your-fish-assay-simple-fixes-for-reducing-high-background-signal/]
- 61. Minimizing off-target signals in RNA fluorescent in situ ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2879521/]
- 62. 5 ways controls can demystify your Stellarisâ„¢ RNA FISH ... [https://blog.biosearchtech.com/five-ways-controls-can-demystify-your-stellaris-rna-fish-experiment]
- 63. 10 Tips for Optimizing In Situ Hybridization (ISH) [https://www.enzo.com/note/10-tips-for-optimizing-in-situ-hybridization-ish/]
- 64. A simple and fast optical clearing method for whole-mount ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12342960/]
- 65. Fluorescence In Situ Hybridization (FISH) [https://www.thermofisher.com/ar/en/home/life-science/cell-analysis/cellular-imaging/in-situ-hybridization-ish/fluorescence-in-situ-hybridization-fish.html]
- 66. Tetrahedral DNA dendritic nanostructure-enhanced FISH ... [https://www.nature.com/articles/s41467-025-64294-1]
- 67. Optimized RNA ISH, RNA FISH and protein-RNA double ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4126239/]
- 68. A Fast and Efficient Fluorescent In situ Hybridization (FISH) ... [https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2022.878062/full]
- 69. fish检测实验æ¥éª¤åŠåº”用-è¡Œä¸šåŠ¨æ€ [https://www.genecreate.cn/industry_news/2644.html]
- 70. Chronic lymphocytic leukemia (CLL) screening and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11897095/]
- 71. Protocol optimization improves the performance of multiplexed ... [https://pubmed.ncbi.nlm.nih.gov/40887478/]
- 72. Isotropic, aberration-corrected light sheet microscopy for ... [https://www.nature.com/articles/s41587-025-02882-8]
- 73. How do I reduce high background in my FISH assay? [https://www.ogt.com/us/resources/fish-resources-and-support/fish-resources/optimize-your-fish-assay-simple-fixes-for-reducing-high-background-signal/]
- 74. Preclinical validation of fluorescence in situ hybridization ... [https://www.sciencedirect.com/science/article/pii/S1098360021034031]
- 75. Fluorescent in situ Hybridization (FISH) [https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/fish-procedure?srsltid=AfmBOoqakRYwu7ZoqWmiDrUOum4OlNP_FPZOi6BfOVByFNz1nZdQHdXV]
- 76. Method validation and analysis of halogenated natural ... [https://pubmed.ncbi.nlm.nih.gov/41062860/]
- 77. Application of Fluorescence In Situ Hybridization (FISH) ... [https://www.mdpi.com/2076-0817/11/12/1450]
- 78. Genomic aberration detection by fluorescence in situ ... [https://www.sciencedirect.com/science/article/abs/pii/S0046817725001935]
- 79. Guidance for Fluorescence in Situ Hybridization Testing ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1867444/]
- 80. Integrating taxonomic and phenotypic information through ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12323603/]
- 81. Evaluation of fluorescent in-situ hybridization technique for ... [https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2875-x]
- 82. Specific cultivation-independent enumeration of viable ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2024.1410709/full]
- 83. Fluorescence In Situ Hybridization Techniques and ... [https://www.nature.com/research-intelligence/nri-topic-summaries/fluorescence-in-situ-hybridization-techniques-and-applications-micro-17216]
- 84. Specific cultivation-independent enumeration of viable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11199854/]
- 85. A Comparison of Molecular Methods for Microbial Testing [https://krakensense.com/blog/a-comparison-of-molecular-methods-for-microbial-testing]
- 86. Comparison of PCR, Fluorescent in Situ Hybridization and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7256870/]
- 87. Article A Comparative Analysis of FISH, RT-PCR, PCR, and ... [https://www.sciencedirect.com/science/article/pii/S0893395222048797]
- 88. Comparison of PCRâ€Based Methods for the Detection ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12447540/]
- 89. Nested-PCR vs. RT-qPCR: A Sensitivity Comparison in the ... [https://www.mdpi.com/2673-8856/4/3/19]
- 90. Comparison of nucleic acid-based detection methods for ... [https://www.sciencedirect.com/science/article/abs/pii/S0044848625001048]
- 91. Application of Array-Based Comparative Genomic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1876176/]
- 92. Microarray-based Comparative Genomic Hybridization ... [http://www.nature.com/scitable/topicpage/microarray-based-comparative-genomic-hybridization-acgh-45432]
- 93. Automated Image Analysis for Quantitative Fluorescence In ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1892889/]
- 94. Advances in chromosomal microarray analysis: Transforming ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11847126/]
- 95. a report of the Cancer Genomics Consortium Plasma Cell ... [https://www.nature.com/articles/s41408-025-01286-w]
- 96. Fluorescence in situ hybridization (FISH): History ... [https://www.sciencedirect.com/science/article/pii/S259000721830008X]
- 97. MALDI-TOF MSåœ¨ä¸´åºŠå¾®ç”Ÿç‰©æ ·æœ¬ç›´æŽ¥æ£€æµ‹ä¸çš„应用 [https://www.labmed.cn/instrument/3792.html]
- 98. 使用质谱法对人脑类器官进行分åæˆåƒ [https://www.jove.com/cn/t/66997/molecular-imaging-of-human-brain-organoids-using-mass-spectrometry]
- 99. 16S/18S FISHè§å…‰æŽ¢é’ˆè®¾è®¡åŠæ£€æµ‹æœåŠ¡å¸¸è§é—®é¢˜åŠè§£å†³æ–¹æ¡ˆ [https://www.tinygene.com/fish/fish-solution]
- 100. FISH - PANELS* - Department of Pathology and Laboratory ... [https://lsom.uthscsa.edu/pathology/reference-labs/clinical-molecular-cytogenetics/fish-panels/]
- 101. FISH技术在肿瘤精准诊疗ä¸çš„应用 [https://www.medsci.cn/article/show_article.do?id=62598542645a]
- 102. Abnova:Gene FISH探针&Split FISH探针 ... - æ¯æ—¥ç”Ÿç‰©è¯„论 [https://www.bio-review.com/abnova%EF%BC%9Agene-fish%E6%8E%A2%E9%92%88split-fish%E6%8E%A2%E9%92%88translocation-fish%E6%8E%A2%E9%92%88%E4%BA%9A%E7%AB%AF%E7%B2%92%E4%B8%8E%E6%9F%93%E8%89%B2%E4%BD%93%E6%8E%A2%E9%92%88/]
- 103. Comparison of Fluorescence in Situ Hybridization and ... [https://www.sciencedirect.com/science/article/pii/S0006497119399100]
- 104. Immunohistochemistry (IHC) Workflow [https://www.rockland.com/resources/immunohistochemistry-workflow/?srsltid=AfmBOoprghuKyJgI86I9ASghd4KdkAKwScUXMLgatjudRKa75lUHoF6E]
- 105. FISH Techniques for Biofilm Detection [https://www.creative-bioarray.com/support/fish-techniques-for-biofilm-detection.htm]
- 106. Optimized workflow for digitalized FISH analysis in pathology [https://pmc.ncbi.nlm.nih.gov/articles/PMC8114497/]